Published online May 15, 2017. doi: 10.4291/wjgp.v8.i2.59
Peer-review started: February 8, 2017
First decision: March 9, 2017
Revised: April 12, 2017
Accepted: April 23, 2017
Article in press: April 24, 2017
Published online: May 15, 2017
Processing time: 109 Days and 9 Hours
To assess whether higher sensitivity of colonic epithelium to hypoxia at the serosal side is associated with oxygen transfer asymmetry.
Rats were fed either with normal chow or a low-sodium diet. Tissues were mounted as flat sheets in a modified, airtight Ussing chamber with oxygen meters in each hemichamber. Mucosal samples from normal diet animals were studied under control conditions, in low-chloride solution and after adding chloride secretion inhibitors and chloride secretagogues. Samples from sodium-deprived rats were studied before and after ouabain addition. In separate experiments, the correlation between short-circuit current and oxygen consumption was analyzed. Finally, hypoxia was induced in one hemichamber to assess the relationship between its oxygen content and the oxygen pressure difference between both hemichambers.
In all studied conditions, oxygen consumption was larger in the serosal hemichamber than in the mucosal one (P = 0.0025 to P < 0.0001). Short-circuit current showed significant correlation with both total oxygen consumption (r = 0.765; P = 0.009) in normoxia and oxygen consumption in the serosal hemichamber (r = 0.754; P = 0.011) during mucosal hypoxia, but not with oxygen consumption in the mucosal hemichamber. When hypoxia was induced in the mucosal hemichamber, an oxygen pressure difference of 13 kPa with the serosal hemichamber was enough to keep its oxygen content constant. However, when hypoxia was induced in the serosal hemichamber, the oxygen pressure difference with the mucosal hemichamber necessary to keep its oxygen content constant was 40 kPa (P < 0.0001).
Serosal oxygen supply is more readily available to support short-circuit current. This may be partly due to a rectifying behavior of transepithelial oxygen transfer.
Core tip: The physiological dependence of the colonic epithelium on oxygen provided from the serosal side is not only due to the structure of its blood supply and the low oxygen pressure of colonic intraluminal contents, since it is also observed in isolated mucosa preparations. This study demonstrates for the first time that a much larger partial pressure difference is needed for oxygen transfer from the mucosal side to the serosal side of the epithelium than for transfer in the opposite direction, a phenomenon that may be considered a rectifying behavior.
- Citation: Saraví FD, Carra GE, Matus DA, Ibáñez JE. Rectification of oxygen transfer through the rat colonic epithelium. World J Gastrointest Pathophysiol 2017; 8(2): 59-66
- URL: https://www.wjgnet.com/2150-5330/full/v8/i2/59.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v8.i2.59
Hypoxia is considered the earliest factor causing organ damage in intestinal ischemia[1], caused, for example, by hypoperfusion associated with septic shock[2], general anesthesia[3], or ischemic colitis; the latter condition is the most frequent form of ischemic injury to the gastrointestinal tract[4,5].
The intestinal epithelium is very sensitive to hypoperfusion. Epithelial ion transport is closely coupled to aerobic metabolism[6,7]. However, the intestinal epithelium normally has relatively low oxygen partial pressure (P
While oxygen consumption by intraluminal bacteria may partially account for the differences, it has been reported that intracolonic P
In most studies of epithelial biology employing Ussing chambers, both sides of the samples are oxygenated. If unremoved, in the intestinal epithelium both the adherent mucus gel layer and the submucosal tissue restrain oxygen diffusion[14]. Hypoxia induced in the serosal hemichamber while keeping a high oxygen pressure at the mucosal side lowers short-circuit current (Isc) just as effectively and with the same time-course as hypoxia simultaneously induced in both sides. On the other hand, hypoxia induced in the mucosal hemichamber does not reduce Isc as long as the serosal side remained oxygenated[15]. Furthermore, non-everted colonic sacs oxygenated from the serosal side showed a 127% higher Isc than everted sacs oxygenated from the mucosal side[15]. One possible cause of the observed asymmetry is higher serosal than mucosal oxygen consumption (QO2); another is some kind of barrier, present at the mucosal but not at the serosal side, which hinders oxygen diffusion.
Our working hypotheses were, first, that the observed asymmetries are due to an intrinsic property of the epithelium, and therefore they should be found under different experimental treatments. Second, that the epithelial hindrance to oxygen diffusion may be different for transfer from lumen to interstitium than in the reverse direction. This may be deemed a rectifying behavior by analogy to electrical devices. Rectification is a property of diodes, which are electrical devices that allow current flow in one direction far more easily than in the other. Some membrane ion channels display rectifying behavior, since they show a nonlinear relationship between the driving force (potential difference) and the resulting current[16].
The aims of this paper are, first, to assess the differences in oxygen consumption provided from the apical side of the epithelium and from its serosal side during different experimental treatments and, second, to measure the partial pressure difference needed for oxygen transfer from the mucosal to the serosal side and for oxygen transfer in the opposite direction.
Wistar-Hokkaido male rats weighing 250-300 g were housed and managed according to the guidelines for animal care and biosafety of our Medical School. The animals were kept at an environmental temperature of 25 °C± 1 °C with a 12-h light-dark cycle.
All animals were given food and drink ad libitum. A group of rats drank tap water and ate a standard rat chow containing 1.77 mg of sodium per gram of food (76.9 mEq/kg; Cargill Co.). A second group was given distilled water and a low-sodium diet with 0.161 mg of sodium per gram of food (7 mEq/kg; ICN Flow Catalog # 902902) for 10 d. The low-sodium diet was purchased from ICN, Inc. (Costa Mesa, California, United States). Daily food consumption was recorded in both groups.
All procedures involving the care and use of animals were approved by the Committee for Animal Care and Use of the Faculty of Medical Sciences, National University of Cuyo. This study was reviewed and approved by the Secretaría de Ciencia y Técnica, National University of Cuyo, institutional review board.
Blood samples from all rats fed with the sodium-deficient diet and an equal number of rats fed with standard chow were obtained during the surgical procedure. Blood was allowed to clot; serum was extracted and frozen at -70 °C. Aldosterone concentration in stored sera was afterwards measured with a coated-tube radioimmunoassay (Diagnostic Products Corporation, Los Angeles, California, United States).
Under ether anesthesia, the entire colon was excised. A 3-cm segment was cut from the descending portion just above the pelvic brim and dissected to obtain an isolated mucosa preparation, as previously described[15]. The segment was cut open along its mesenteric border, the adherent mucus gel layer was gently removed with a cotton tip soaked in dissecting solution and the segment was mounted as a flat sheet in a modified Ussing chamber (described below).
The composition of the standard Ringer solution employed was as follows (in mmol/L): Na 132.8; K 4.5; Ca 1.25; Mg 1.0; Cl 114.0; HCO3 24.0; H2PO4 1.0; SO4 1.0; D (+) glucose 10.0. In the low-chloride solution, most chloride was replaced by sulfate, with the addition of mannitol to compensate for the difference in osmolality[11], as follows (in mmol/L): Na 136.6; K 4.5; Ca 1.25: Mg 1.0; Cl 2.5; HCO3 24.0; H2PO4 2.0; SO4 58.1; D (+) glucose 10.0; mannitol 93.4. The osmolality 0f both solutions was 280 mOsm/kg H2O.
Two gas mixtures were used: 950 mL/L O2 - 50 mL/L CO2 (normoxic mixture) and 950 mL/L N2 - 50 mL/L CO2 (hypoxic mixture). The gas mixtures were purchased from Air Liquide, Inc. (Buenos Aires, Argentina). Their compositions were certified by the company. When gassed with either gas mixture, the pH of both solutions was 7.40.
Bumetanide, 3-methyl-1-isobutilxanthine (IBMX), serotonin, and amiloride were purchased from Sigma-Aldrich (St Louis, Missouri, United States), and diphenylamine-2-carboxylate (DPC) from ICN, Inc (Costa Mesa, California, United States). The appropriate drug solutions were prepared just before each experiment. The standard Ringer solution was employed to dissolve serotonin and carbachol; dimethylsulfoxide was used for IBMX and bumetanide, and absolute ethanol was used for amiloride and DPC. As indicated, bumetanide, IBMX and serotonin were added to the serosal hemichamber, while DPC and amiloride were added to the mucosal hemichamber to achieve the following concentrations (in mmol/L): bumetanide 0.1; DPC 0.5; IBMX 0.1; serotonin 0.1, and amiloride 0.1. At the start of each experiment, 91 μg/mL gentamycin (Schering-Plough, Buenos Aires, Argentina) was added to each hemichamber, to prevent bacterial overgrowth.
For this report, a modified Ussing chamber which has already been described and validated was used[17]. The chamber is airtight and it has a 1 cm2 window between the hemichambers. Polarimetric oxygen meters (CellOx 325, WTW GmbH, Weilheilm, Germany) are attached to each hemichamber. This arrangement allows measurement of the oxygen concentration and its time course in both the hemichamber facing the basolateral (serosal) side of the epithelium and the hemichamber facing its apical (mucosal) side. The chamber content was kept at 37 °C with a water jacket fed from a thermostatic reservoir.
The transepithelial potential difference was recorded with calomel electrodes connected to each hemichamber through saline bridges (agar-in-Ringer, 30 g/L). Ag-AgCl electrodes were used to supply current to the chamber from an amplifier, in order to clamp the transepithelial potential difference (TPD) at 0 mV. The output of the amplifier took into account corrections for solution resistance and bridge asymmetry. The short-circuit condition was kept throughout all experiments, except for the brief periods of release needed to measure open circuit TPD. The transepithelial resistivity was calculated, according to Ohm’s law, as the quotient between TPD and Isc.
Before each experiment, oxygen probes were calibrated according to the user’s manual and had their slopes checked and recorded. At the beginning of each experiment, the hemichambers were gassed with the normoxic mixture until a plateau of their oxygen concentration was reached. Afterwards both hemichambers were hermetically closed. QO2 was measured under baseline conditions for 30-min periods, starting 60 min after the closure of the hemichambers.
Solution replacement was carried out without opening the chamber with a gravity driven system which allowed passing from a water-jacketed reservoir (37 °C) through the chamber a volume of low-chloride solution 10-fold larger than the chamber volume, with the overflow being drained through siphons. In experiments involving replacement of Ringer with low-chloride solution or addition of chloride secretion blockers, Isc was allowed to stabilize during 20 min before QO2 was measured for the next 30 min. On the other hand, the 30-min QO2 measurement was started immediately after addition of IBMX or serotonin.
The change in oxygen concentration in each hemichamber during the measurement period was used to calculate QO2, as detailed elsewhere[11]. In experiments assessing oxygen transfer between the hemichambers, a baseline QO2 was obtained after attaining the same oxygen level in both hemichambers. Then hypoxia was induced in one hemichamber by gassing its contents with 950 mL/L N2 - 50 mL/L CO2 for various times while bubbling 950 mL/L O2 - 50 mL/L CO2 in the other hemichamber, to obtain graded differences in oxygen pressure (∆P
After a 15-min period of reoxygenation of both hemichambers, the procedure described above was repeated for inducing hypoxia in the other hemichamber. The ∆P
After each experiment, the mucosa was replaced with a polyethylene membrane for a blankrun. The experiment was discarded if the decrease in oxygen concentration was above 5% of that observed with the biological sample.
QO2 in the serosal and mucosal hemichambers and Isc under each tested condition were compared with a paired, two-sided Student’s t test. An unpaired, two-sided Student’s t test was employed for analysis of food intake, sodium intake, and serum aldosterone in rats fed with normal sodium diet and in those submitted to the low-sodium diet. During analysis, significant deviations from a Gaussian distribution were ruled out with the Kolmogorov-Smirnov test.
A one-way analysis of variance with Geisser-Greenhouse correction was used to assess differences in the magnitude of the serosal vs mucosal difference in QO2 under all conditions tested. Simple linear regression was used to assess the relationships between Isc and ∆P
A commercial software was employed for statistical analyses (GraphPad Prism version 5.1 for Windows, GraphPad Software, San Diego, California, ∆). Values are reported as means ±
Mean daily food intake of rats fed with the low-sodium diet (80.0 ± 1.4 g per kilogram body weight; n = 6) was not significantly different from that of rats given standard chow (82.1 ± 0.9 g per kilogram body weight; n = 20). The respective mean daily sodium intakes per kilogram body weight were 12.9 ± 0.3 mg and 145.3 ± 1.6 mg (P < 0.0001). As expected, serum aldosterone concentration was significantly higher in rats with low sodium intake (10.49 ± 2.1 nmol/L; n = 6) than in controls (1.42 ± 0.26 nmol/L; n = 6, P = 0.0016).
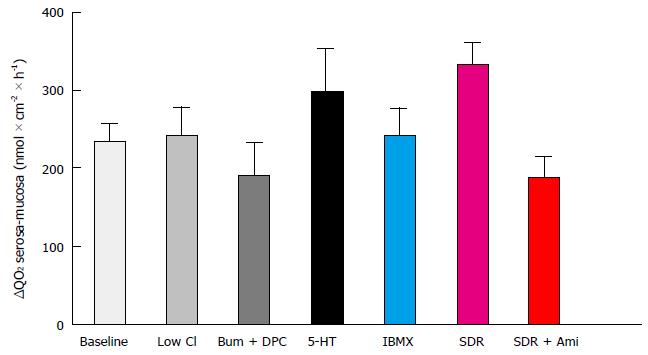
Values of Isc, total QO2, serosal QO2, and mucosal QO2 for epithelial samples are shown in Table 1. Compared with controls, Isc and QO2 were higher in the presence of chloride secretagogues and in epithelial samples from sodium-deprived rats. Conversely, Isc and QO2 were lower in low-chloride solution, in the presence of chloride secretion blockers or, in tissues from sodium-deprived animals, after addition of amiloride. Under all conditions tested, serosal oxygen consumption was higher than mucosal oxygen consumption (Figure 1). The magnitude of this difference was similar for all treatments (P = 0.0847 according to one-way analysis of variance).
| Treatment | n | Isc μmol × cm-2 × h-1 | Total QO2 μmol × cm-2 × h-1 | Serosal QO2 μmol × cm-2 × h-1 | Mucosal QO2 μmol × cm-2 × h-1 |
| None | 20 | 3.33 ± 0.21 | 2.79 ± 0.06 | 1.52 ± 0.04 | 1.28 ± 0.08b |
| Low Cl | 6 | 0.66 ± 0.07 | 2.56 ± 0.13 | 1.41 ± 0.08 | 1.14 ± 0.06d |
| Bum + DPC | 6 | 0.63 ± 0.08 | 2.47 ± 0.15 | 1.35 ± 0.07 | 1.15 ± 0.08d |
| Serotonin | 6 | 5.30 ± 0.29 | 3.64 ± 0.13 | 1.97 ± 0.08 | 1.67 ± 0.06d |
| IBMX | 6 | 3.98 ± 0.24 | 3.22 ± 0.09 | 1.75 ± 0.07 | 1.47 ± 0.06d |
| SDR | 6 | 8.50 ± 0.45 | 3.71 ± 0.35 | 2.02 ± 0.15 | 1.68 ± 0.12b |
| SDR + Ami | 6 | 0.33 ± 0.05 | 2.88 ± 0.12 | 1.53 ± 0.07 | 1.35 ± 0.08d |
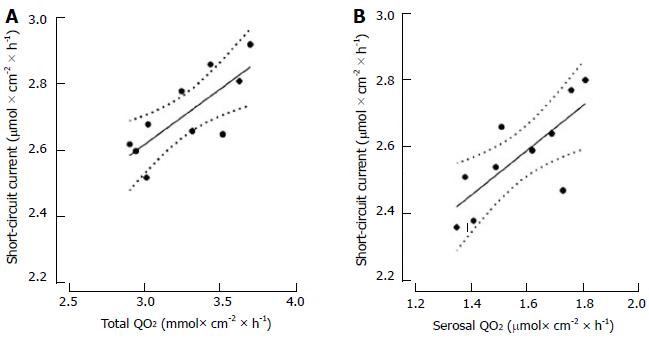
Isc was correlated with total QO2 and serosal QO2 both under baseline condition and during mucosal hypoxia (Figure 2), but those correlations were lost during serosal hypoxia. On the other hand, Isc was not correlated with mucosal QO2 under any condition (Table 2).
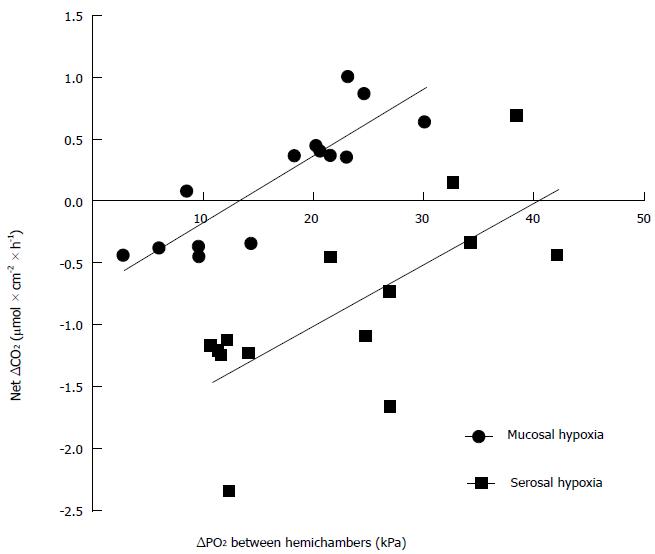
When different oxygen tension differences were imposed between both hemichambers, the oxygen content of the hypoxic hemichamber increased with time when ∆P
Present results show that, when oxygen is available from both the serosal and the mucosal sides to distal colonic epithelium, the tissue consumes more oxygen supplied from the serosal than from the mucosal side. Since blood supply plays no role in oxygen availability under present in vitro conditions, the observed preference may not be explained by the path through which oxygen is normally provided to the epithelium. Furthermore, the higher serosal QO2 was observed under a variety of conditions, including those in which chloride secretion is the major electrogenic phenomenon and those in which the major electrogenic phenomenon is sodium absorption. This suggests that the asymmetry is also not due to the specific ion being transported but represents a physiological characteristic of the colonic epithelium. It should be noted that this asymmetry has also been demonstrated in epithelial samples from human sigmoid colon[18].
The importance of oxygen supply from the serosal side to sustain electrogenic transport was further corroborated by the correlation between Isc and serosal QO2 under baseline condition and during hypoxia induced in the mucosal hemichamber, while no such correlation was present during hypoxia induced in the serosal hemichamber. On the other hand, no correlation was found between Isc and mucosal QO2 in any condition tested.
When graded hypoxia is induced in one hemichamber, while the other is saturated with oxygen, theoretically the oxygen content of the hypoxic hemichamber could decrease, remain constant, or increase with time. It would decrease if the hypoxic chamber still provides part of the oxygen consumed by the epithelium. It would remain constant if all oxygen consumed by the epithelium is provided by the oxygenated chamber and there is no net oxygen transfer between the hemichambers. Finally, it would actually increase if the oxygenated hemichamber, apart from providing all the oxygen that the epithelium consumes, transfers part of its oxygen to the hypoxic hemichamber. The relationship between ∆CO2 in the hypoxic hemichamber and ∆P
One explanation for the larger contribution of oxygen supply from the serosal side may be that the oxygen permeability of the basolateral membrane to oxygen is larger than the oxygen permeability of the apical membrane. While it is classically accepted that biological membranes are very permeable to gases, low permeability of some membranes to gases such as ammonia or carbon dioxide has been reported, for example in gastric glands[19] and colonic epithelium[20]. This issue has been recently reviewed, and the existence of gas channels has been postulated[21,22]. There are few reports on membrane oxygen permeability[21] and the results are contradictory[22].
Even if apical and basolateral epithelial membranes had different oxygen permeability, this would not explain per se the apparent rectifying behavior of oxygen transfer. We are not aware of reports on this topic for oxygen, or for any other gas. Since gases generated in the colon as products of bacterial fermentation are partly transferred into the bloodstream[10], it is of physiological and clinical interest to assess whether their rate of transfer from the lumen to the interstitium is selectively limited, as it seems to be the case for oxygen.
In conclusion, the sigmoid colon epithelium in vitro in a rat model preferentially consumes oxygen supplied from the serosal side. Isc correlates with serosal oxygen consumption, but not mucosal oxygen consumption. The ∆P
The colonic epithelium has a high oxygen consumption, a large part of which is needed to sustain electrogenic ion transport. In vivo, oxygen is mostly supplied from the serosal side, since the luminal environment has a very low oxygen partial pressure. When the epithelium is placed in an Ussing chamber, oxygen is usually available from both sides of the epithelium, but even then, the serosal supply is more important to sustain electrogenic ion transport than the mucosal supply.
The intestinal epithelium is normally submitted to a relatively low oxygen pressure when compared with other tissues, a condition known as physiological hypoxia. This makes the epithelium susceptible to hypoxic injury when the oxygen supply is further compromised by disease.
In this paper, it is shown that serosal supply provides more oxygen than mucosal supply to the colonic epithelium under several different conditions, suggesting that the difference is an intrinsic property of the tissue. For the first time, evidence is provided suggesting that transepithelial oxygen diffusion from serosa to mucosa needs a lower gradient of oxygen pressure than diffusion from mucosa to serosa.
Since epithelial hypoxia seems to be an important cause of injury and dysfunction in several conditions affecting the intestinal epithelium, a deeper knowledge of the factors influencing epithelial oxygen supply may help to understand their pathophysiology and to devise better management strategies.
Rectification: A term borrowed from electronics, referring to devices such as diodes, which allow electric current to flow more easily in one direction than in the opposite one. In the present context, it is applied to the observation that oxygen diffuses more easily from the serosal to the mucosal side of the epithelium than in the opposite direction. Short-circuit current: The electrical current passing through the epithelium needed to keep the transepithelial potential difference at 0 mV. It is a measure of electrogenic ion transport.
The aim of this study was to assess whether higher sensitivity of colonic epithelium to hypoxia at the serosal side is associated with oxygen transfer asymmetry. It is a very well design study and the issue is elegantly developed.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Huerta-Franco MR, Koch TR S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | MacDonald PH. Ischaemic colitis. Best Pract Res Clin Gastroenterol. 2002;16:51-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | van Haren FM, Sleigh JW, Pickkers P, Van der Hoeven JG. Gastrointestinal perfusion in septic shock. Anaesth Intensive Care. 2007;35:679-694. [PubMed] |
| 3. | Leung FW. Endoscopic reflectance spectrophotometry and visible light spectroscopy in clinical gastrointestinal studies. Dig Dis Sci. 2008;53:1669-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Theodoropoulou A, Koutroubakis IE. Ischemic colitis: clinical practice in diagnosis and treatment. World J Gastroenterol. 2008;14:7302-7308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 159] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 5. | Feuerstadt P, Brandt LJ. Update on Colon Ischemia: Recent Insights and Advances. Curr Gastroenterol Rep. 2015;17:45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Mandel LJ, Balaban RS. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol. 1981;240:F357-F371. [PubMed] |
| 7. | Del Castillo JR, Ricabarra B, Sulbarán-Carrasco MC. Intermediary metabolism and its relationship with ion transport in isolated guinea pig colonic epithelial cells. Am J Physiol. 1991;260:C626-C634. [PubMed] |
| 8. | Zheng L, Kelly CJ, Colgan SP. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am J Physiol Cell Physiol. 2015;309:C350-C360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 337] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 9. | Suarez F, Furne J, Springfield J, Levitt M. Insights into human colonic physiology obtained from the study of flatus composition. Am J Physiol. 1997;272:G1028-G1033. [PubMed] |
| 10. | Sahakian AB, Jee SR, Pimentel M. Methane and the gastrointestinal tract. Dig Dis Sci. 2010;55:2135-2143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Bornside GH, Donovan WE, Myers MB. Intracolonic tensions of oxygen and carbon dioxide in germfree, conventional, and gnotobiotic rats. Proc Soc Exp Biol Med. 1976;151:437-441. [PubMed] |
| 12. | Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano RN, Lewis JD, Li H. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147:1055-1063.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 620] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 13. | Guebel DV, Torres NV. A computer model of oxygen dynamics in human colon mucosa: implications in normal physiology and early tumor development. J Theor Biol. 2008;250:389-409. [PubMed] |
| 14. | Saldeña TA, Saraví FD, Hwang HJ, Cincunegui LM, Carra GE. Oxygen diffusive barriers of rat distal colon: role of subepithelial tissue, mucosa, and mucus gel layer. Dig Dis Sci. 2000;45:2108-2114. [PubMed] |
| 15. | Saraví FD, Saldeña TA, Cincunegui LM, Carra GE. Asymmetrical oxygen availability from serosal and luminal sides of rat distal colon epithelium. Rev Esp Fisiol. 1997;53:367-375. [PubMed] |
| 16. | Siegelbaum SA, Koester J. Ion channels. Principles of neural science. New York: McGraw-Hill Medical 2013; 100-125. |
| 17. | Saraví FD, Saldeña TA, Carrera CA, Ibáñez JE, Cincunegui LM, Carra GE. Oxygen consumption and chloride secretion in rat distal colon isolated mucosa. Dig Dis Sci. 2003;48:1767-1773. [PubMed] |
| 18. | Carra GE, Ibáñez , Saraví FD. Electrogenic transport, oxygen consumption, and sensitivity to acute hypoxia of human colonic epithelium. Int J Colorectal Dis. 2011;26:1205-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Boron WF, Waisbren SJ, Modlin IM, Geibel JP. Unique permeability barrier of the apical surface of parietal and chief cells in isolated perfused gastric glands. J Exp Biol. 1994;196:347-360. [PubMed] |
| 20. | Endeward V, Gros G. Low carbon dioxide permeability of the apical epithelial membrane of guinea-pig colon. J Physiol. 2005;567:253-265. [PubMed] |
| 21. | Boron WF. Sharpey-Schafer lecture: gas channels. Exp Physiol. 2010;95:1107-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Endeward V, Al-Samir S, Itel F, Gros G. How does carbon dioxide permeate cell membranes? A discussion of concepts, results and methods. Front Physiol. 2014;4:382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |