Copyright
©The Author(s) 2016.
World J Gastrointest Pathophysiol. Feb 15, 2016; 7(1): 27-37
Published online Feb 15, 2016. doi: 10.4291/wjgp.v7.i1.27
Published online Feb 15, 2016. doi: 10.4291/wjgp.v7.i1.27
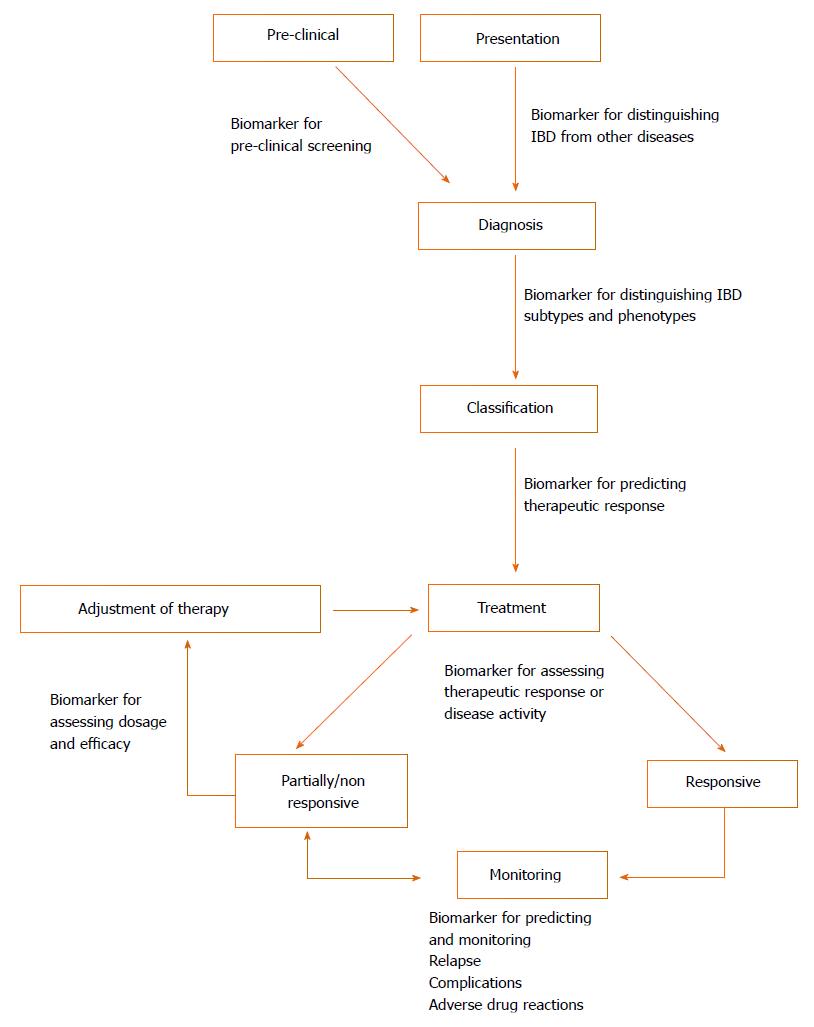
Figure 1 Potential application of biomarkers in inflammatory bowel disease in different stages of clinical management.
When presenting clinically, one important use of biomarkers could be in the diagnosis of IBD, as well as differentiating subtypes (e.g., UC vs CD) and phenotypes (e.g., fistulising). Whilst not currently part of management, preclinical screening for IBD may be a possibility. Biomarkers can also be used to predict response to therapy and objectively measure therapeutic response and disease severity. Due to the relapsing and remitting course of IBD, monitoring is necessary for assessing relapse, adverse outcomes and complications (e.g., strictures, fistulas and colorectal cancer). Most of these aspects necessitate endoscopic procedures and would benefit from biomarker substitutes. CD: Crohn’s disease; UC: Ulcerative colitis; IBD: Inflammatory bowel disease.
- Citation: Chan PP, Wasinger VC, Leong RW. Current application of proteomics in biomarker discovery for inflammatory bowel disease. World J Gastrointest Pathophysiol 2016; 7(1): 27-37
- URL: https://www.wjgnet.com/2150-5330/full/v7/i1/27.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v7.i1.27