Published online Oct 15, 2011. doi: 10.4291/wjgp.v2.i5.72
Revised: August 12, 2011
Accepted: August 19, 2011
Published online: October 15, 2011
Inflammation and immune activation in the gut are usually accompanied by alteration of gastrointestinal (GI) motility. In infection, changes in motor function have been linked to host defense by enhancing the expulsion of the infectious agents. In this review, we describe the evidence for inflammation and immune activation in GI infection, inflammatory bowel disease, ileus, achalasia, eosinophilic esophagitis, microscopic colitis, celiac disease, pseudo-obstruction and functional GI disorders. We also describe the possible mechanisms by which inflammation and immune activation in the gut affect GI motility. GI motility disorder is a broad spectrum disturbance of GI physiology. Although several systems including central nerves, enteric nerves, interstitial cells of Cajal and smooth muscles contribute to a coordinated regulation of GI motility, smooth muscle probably plays the most important role. Thus, we focus on the relationship between activation of cytokines induced by adaptive immune response and alteration of GI smooth muscle contractility. Accumulated evidence has shown that Th1 and Th2 cytokines cause hypocontractility and hypercontractility of inflamed intestinal smooth muscle. Th1 cytokines downregulate CPI-17 and L-type Ca2+ channels and upregulate regulators of G protein signaling 4, which contributes to hypocontractility of inflamed intestinal smooth muscle. Conversely, Th2 cytokines cause hypercontractilty via signal transducer and activator of transcription 6 or mitogen-activated protein kinase signaling pathways. Th1 and Th2 cytokines have opposing effects on intestinal smooth muscle contraction via 5-hydroxytryptamine signaling. Understanding the immunological basis of altered GI motor function could lead to new therapeutic strategies for GI functional and inflammatory disorders.
- Citation: Akiho H, Ihara E, Motomura Y, Nakamura K. Cytokine-induced alterations of gastrointestinal motility in gastrointestinal disorders. World J Gastrointest Pathophysiol 2011; 2(5): 72-81
- URL: https://www.wjgnet.com/2150-5330/full/v2/i5/72.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v2.i5.72
Intestinal inflammation and immune activation are accompanied by alteration of gastrointestinal (GI) motility, associated with altered function of enteric nerves, intestinal cell of Cajal (ICCs) or smooth muscles. Changes in motor function have been described in experimental models following a variety of inflammatory stimuli, including infection[1,2], chemical irritation[3,4] and immune activation[5,6]. In the context of infection, changes in motor function have been linked to host defense by enhancing the expulsion of the infectious agent. Also, evidence has emerged in animal studies that low-grade inflammation in the gut could alter GI motor function[6,7].
From a clinical viewpoint, some motility disorders have been associated with evidence of immune activation, such as inflammatory bowel disease (IBD), ileus, achalasia, functional GI disease (FGID), or life-threatening intestinal pseudo-obstruction[8]. An understanding of the mechanisms that underlie immune-mediated changes in gut motor function is therefore critical, not only in understanding the pathophysiology of, but also in devising new therapeutic strategies for, these disorders.
This review describes the evidence for immune activation in GI inflammation, infection and FGID, with a particular focus on cytokine-induced alteration of GI motility.
Common symptoms of GI diseases are abdominal pain or discomfort, diarrhea, constipation, fullness and bloating. A mechanical approach to constipation consists of poor intake of fluid or fiber, slow colonic transit, and outlet dysfunction in the anorectal area. Diarrhea is an increase in the volume of stool or frequency of defecation, and is categorized into osmotic, secretory, exudative, and altered intestinal motility. Acute diarrhea that lasts for < 14 d is usually related to a bacterial, viral, or parasitic infection and poses the risk of dehydration. Chronic diarrhea that lasts at least 4 wk is more likely to be due to alterations in GI motility and rapid transit than to a secretory component[9]. The symptoms of GI disorders reflect a broad spectrum of disturbance of GI physiology, including altered epithelial, muscle, intestinal and enteric neural function and are also, at least in part, due to immune activation.
Several types of bacteria, including: Campylobacter, Salmonella, Shigella and Escherichia coli; viruses, including: Rotavirus, Norwalk virus, Cytomegalovirus and herpes simplex virus (HSV); and parasites, including: Giardia lamblia, Entamoeba histolytica and Cryptosporidium cause diarrhea. Different pathogens such as enterotoxin invade the host and cause infectious diarrhea.
In bacterial infection, Salmonella is a leading cause of GI disease worldwide. Ma et al[10] have reported that tumor necrosis factor (TNF)-α modulates the expression of Salmonella typhimurium effector proteins and enhances interleukin (IL)-8 secretions in intestinal epithelial cells. Other studies have shown that IL-6 may play an important role in triggering systemic immune response against Salmonella[11,12]. Campylobacter jejuni infection, which induces a number of cytokines and chemokines including IL-8 and IL-10[13], is also a common cause of human acute bacterial gastroenteritis.
In IBD such as Crohn’s disease (CD) and ulcerative colitis (UC), there are longstanding observations of altered motility and intestinal muscle contractility[14,15].
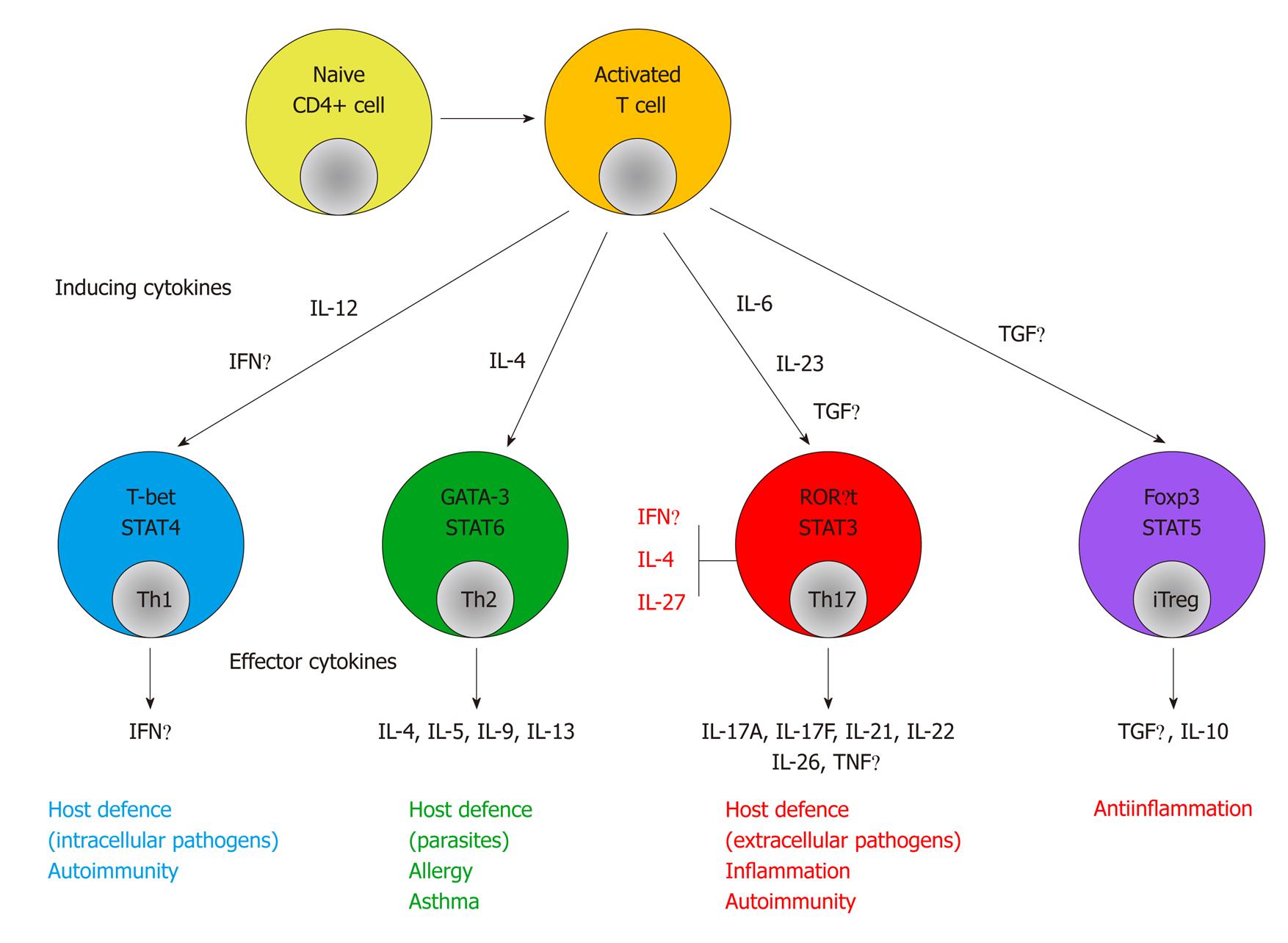
Traditionally, CD has been associated with a T helper (Th)1 cytokine profile. Recent studies have indicated that Th17 cells as well as Th1 cells play a major role in the pathogenesis of CD. Th17 cells express the IL-23 receptor (IL-23R) on their surface. Other studies have identified IL-23R and other genes involved in the differentiation of Th17 cells as IBD susceptibility genes[16-20].
Th17 cells produce IL-17, IL-17F and IL-22, thereby inducing a massive tissue reaction, owing to the broad distribution of IL-17R and IL-22R. Th17 cells also secrete IL-21 to communicate with cells of the immune system. Differentiation factors [transforming growth factor (TGF)β plus IL-6 or IL-21], growth and stabilization factor IL-23 and transcription factors [signal transducer and activator of transcription (STAT)3, retinoid-related orphan receptor (ROR)γt and RORα] have recently been identified as involved in the development of Th17 cells[21].
Some studies have shown delays in gastric and intestinal transit that cannot be accounted for on the basis of mechanical obstruction, and are therefore likely due to inflammation-induced alterations in the motility apparatus[22-25]. Conversely, our groups have shown previously that contractility of intestinal smooth muscle strips and cells from the inflamed intestine of CD patients exhibit increased contractility in vitro after stimulation by carbachol[14,26]. Although CD is well recognized as having a Th1-dominant cytokine profile, we have demonstrated the dominant expression of the Th2 cytokine IL-4, with little change in the Th1 cytokine interferon (IFN)γ in the muscularis externa of small intestinal segments from CD patients. We have found that Th2 cytokines, IL-4 and IL-13 enhance muscle cell contractility in humans and mice[26,27], and IL-17 enhances muscle cell contractility in mice (unpublished observations), therefore, there is the possibility that Th2 or Th17 immune activation alters muscle contractility in CD patients.
UC is characterized by an exaggerated Th2-like response as demonstrated by increased production of Th2 cytokines such as IL-4, IL-5 and IL-13[28,29]. TNF-α mRNA is highly expressed in colon biopsy from UC patients correlating with the grade of inflammation[30]. Five genes involved in downstream signaling of IL-23R, IL-12B, Janus kinase 2, STAT3 and IL-2b mediate susceptibility to UC. These findings suggest that Th17 cells are also involved in UC pathogenesis[17-19,31]. Kobayashi et al[32] have demonstrated significant upregulation of IL-17A in lamina propria CD4+ T cells following IL-23 stimulation in UC. It has been reported that high expression levels of the Th17 cytokines IL-17A, IL-22 and IL-26 are found in the inflamed colon of CD patients and in active UC[33-35].
Altered colonic motor function in UC has been well documented[36-38]. Terry et al[39] reported that melatonin, which is an important regulator of GI inflammation and motility, might have an ameliorative effect on UC. Ohama et al[40,41] have shown that protein kinase C (PKC)-potentiated phosphatase inhibitor protein-17 kDa (CPI-17) expression is decreased in smooth muscle from UC patients. CPI-17 is downregulated by IL-1β and might contribute to the decreased motor function.
Ileus occurs as a result of hypomotility of the GI tract in the absence of mechanical bowel obstruction. Presumably, the muscle of the bowel wall is transiently impaired and fails to transport intestinal contents. This lack of coordinated propulsive action leads to the accumulation of gas and fluids within the bowel. Many factors cause ileus, such as sepsis, drugs, trauma and GI inflammation, and most cases of ileus occur after abdominal surgery. The mechanisms underlying the development of postoperative ileus are complex, and involve central neural reflexes, hormonal influences, local molecular inflammatory responses and the recruitment into the intestinal muscularis of activated immune cells[42-46]. Immune activation is involved in ileus as well as IBD. Serum IL-6 and IL-1β are increased in patients with ileus[47].
Bauer's group[48-51] have shown from animal studies that surgical manipulation of the intestine activates the dense network of normally quiescent macrophages, as demonstrated by phosphorylation of mitogen-activated protein kinases (MAPKs) with resultant activation of transcription factors, early growth response gene-1, nuclear factor κB (NF-κB), IL-6 and STAT3. The translocation of the transcription factors to the nucleus ultimately induces the secretion of a complex inflammatory milieu of proinflammatory cytokines: TNF-α, IL-1β and IL-6, and chemokines. Furthermore, NO and prostaglandins have the important role of smooth muscle inhibition in postoperative ileus.
Esophageal achalasia is a motor disorder that is characterized by the absence of esophageal peristalsis and by incomplete relaxation of the lower esophageal sphincter (LES). The failure of LES relaxation is primarily caused by the loss of the inhibitory innervation of the esophageal myenteric plexus[52].
Recent evidence has shown that HSV-1 is involved in the pathogenesis of achalasia[53]. Facco et al[54] reported that achalasia patients are characterized by significantly higher esophageal lymphocyte infiltration, mainly represented by CD3+CD8+ T cells than controls. LES-infiltrating lymphocytes recognize HSV-1 antigens specifically. Facco et al[54] observed that IL-1β, IFNγ and IL-2 are increased in achalasia patients. Another group has shown that in the immune activation of achalasia patients, TNF-α is significantly increased in the LES[55].
Eosinophilic esophagitis is an important and established cause of dysphagia, which is caused by exposure to exogenous allergens. Eosinophils and IL-5 produced by Th2 cytokines play a crucial role in this disease. Patients are exposed to food or air allergens. Antigen presenting cells (APCs) process these antigens and present them to Th2 cells. Activated Th2 cells produce IL-5, which is crucial for the terminal differentiation and proliferation of eosinophils. IL-4, also produced by Th2 cells, promotes eosinophilic accumulation and IgE production from B cells. In addition, Th2 cells and activated mast cells release IL-13 and TNF that promote local inflammation. GI epithelial cells produce eotaxins, which have essential chemokine activity for the recruitment of circulating eosinophils to the site of inflammation. As a result, mature eosinophils accumulated in the esophagus, are activated, degranulate and release multiple cytotoxic agents[56].
Microscopic colitis is a common cause of chronic watery diarrhea, especially among older persons. Diagnosis requires histological analysis of colon biopsy samples in the appropriate clinical setting[57]. Microscopic colitis demonstrates a Th1 mucosal cytokine profile with IFNγ as the predominantly upregulated cytokine, with concurrent induction of NO synthase and downregulation of IFNγ-related cell junction proteins[58].
Celiac disease is a disorder that is characterized by a deregulated immune response to ingested wheat gluten and related cereal proteins in susceptible individuals[59,60]. The characteristic features of celiac disease include nausea, bloating and diarrhea in patients presenting with otherwise typical irritable bowel syndrome (IBS)[61]. Several studies have shown increased concentrations of 5-hydroxytryptamine (5-HT) in the duodenal mucosa[62], increased plasma 5-HT levels[63] and increased urine excretion of the 5-HT metabolite and 5-hydroxyindoleacetic acid[64].
It is considered that the onset of celiac disease is mediated by a skewed Th1 response[65]. In recent literature it has been shown that gliadin-specific Th17 cells are present in the mucosa of celiac disease patients. These Th17 cells have a role in the pathogenesis of the disease as they produce pro-inflammatory cytokines (such as IL-17, IFNγ and IL-21), mucosa-protective IL-22 and regulatory TGFβ, which actively modulates IL-17A production by T cells in the celiac mucosa[66].
Chronic idiopathic intestinal pseudo-obstruction (CIIP) is a rare, progressive and life-threatening syndrome that is characterized by severely impaired GI motility. Recurrent episodes of abdominal pain and distention are accompanied by bloating, nausea and vomiting without evidence of mechanical obstruction[67]. CIIP may occur throughout the GI tract, but usually involves the small bowel. Several neurotropic viruses have the ability to infect the central and enteric nervous systems. Selgrad et al[68] and Sanders et al[69] have shown that the polyoma virus, JC virus, infects the enteric glia of patients with CIIP[68]. JC virus may infect ICCs and therefore contribute to ICC loss or to re-differentiation to smooth muscle cells. Further investigation is needed.
FGIDs are common clinical syndromes worldwide. Functional dyspepsia (FD) is characterized by the presence of recurrent or chronic upper abdominal symptoms, such as epigastric pain, early satiety and fullness, without anatomical or biochemical abnormalities[70]. There is increasing evidence for involvement of the immune system in FD. Kindt et al[71] have reported that, compared to controls, stimulated lymphocyte expression of IL-5 and IL-13 is enhanced in IBS, FD and non-cardiac chest pain. Conversely, stimulated monocytic IL-12 and lymphocytic IL-10 expression is reduced in IBS and FD, while IFNγ expression is also reduced in FD patients. A shift towards a Th2 cytokine profile is present in FGID, while the cellular immunophenotype remains largely unchanged. Arisawa et al[72] have reported that IL-17F 7488T and macrophage migration inhibitory factor -173C alleles are significantly associated with the development of FD, particularly epigastric pain syndrome, a subgroup of FD, in Helicobacter pylori-infected subjects.
Futagami et al[73] have reported that gastric emptying evaluated by T-max values in post-infectious FD patients is similar to that in controls. However, the degree of histrogical duodenitis in post-infectious FD is significantly greater than that in controls. CCR2/CD68-double positive cell number in post-infectious FD patients is significantly increased.
IBS is characterized by the presence of abdominal pain or discomfort and an alteration in bowel habits[74]. The pathogenesis is considered to be multifactorial and includes psychosocial factors, visceral hypersensitivity, infection, microbiota and immune activation. Several reports have described increased numbers of T cells in various lymphoid compartments of the small or large intestine in IBS patients[75-78]. Pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α in peripheral blood mononuclear cells[79] and IL-6 and IL-8 in serum[80,81] have been reported to be increased in IBS patients.
Goblet cells: The mucous layer that coats the GI tract is the front line of innate host defense largely because of the secretory products of intestinal goblet cells. In most intestinal infections, induction of goblet cells and mucin synthesis and secretion occur frequently, during the acute phase, to expel antigens[82].
Macrophages: Macrophages perform a key role in innate defense against foreign invaders and produce a number of cytokines such as IL-1β, IL-6 and TNF-α. Macrophages are not crucial for changes in muscle contraction in Trichinella spiralis-infected mice[83]. Innate immune response seems not to have a major role in muscle function.
Adaptive immune response: APCs present antigens to CD4+ Th cells. Th cell-dependent immune responses are divided into four subsets: Th1, Th2, Th17 and T regulatory (Treg). Th1 cells produce IFNγ and their primary role is protection against intracellular microbes. Th2 cells produce IL-4, IL-5 and IL-13 and are involved in allergic disorders and protection against extracellular pathogens. Th1 differentiation is mainly driven by IL-12 and IFNγ, while IL-4 drives Th2 differentiation. Treg cells are important in the control of immune responses to self-antigens, prevention of autoimmunity and maintenance of self-tolerance. In contrast, IL-17-producing Th17 cells play a major role in autoimmunity[19] (Figure 1).
Th1/Th2/5-HT: Recent animal studies have shown that Th1 and Th2 immune response is associated with hypocontractility or hypercontractility of inflamed intestinal smooth muscle, respectively.
We have previously shown[7] that Th1 and Th1-related cytokines cause hypocontractility of inflamed intestinal smooth muscle. TNF-α and IL-1β inhibit carbachol-induced contraction via downregulation of CPI-17[84] and L-type Ca2+ channels[85], respectively. Other groups have shown that surgical manipulation suppresses jejunal contractions with upregulation of IL-6, TNF-α, cyclooxygenase-2 and inducible NO synthase[86]. We also have shown that incubation of IFNγ with intestinal smooth muscle decreases carbachol-induced smooth muscle cell contraction[87]. Wells and Blennerhassett have reported a decrease in muscle contractions in 2,4,6-trinitrobenzenesulphonic acid (TNBS)-inflamed preparations[88]. In a Th1-dominant, TNBS-induced colitis model, it has been shown that carbachol- and 5-HT-induced contractility of rat colonic circular smooth muscle cells is decreased in the acute phase, and 5-HT-mediated contraction is still impaired by day 36 post-TNBS.
On the contrary, the Th2 cytokines IL-4 and IL-13 acting via STAT6 mediate the development of nematode T. spiralis-induced intestinal muscle hypercontractility, which contributes to worm expulsion[27,89,90]. A model of Nippostrongylus brasiliensis infection supports our finding that Th2 responses mediate muscle contraction[91,92]. Ihara et al[93] have shown that MAPK pathways play crucial roles in Th2-cytokine-mediated Ca2+ sensitization and hypercontractility observed in inflamed colonic circular smooth muscle from sodium dextran sulfate-treated mice.
We evaluated the association of 5-HT with Th1/Th2 responses. 5-HT influences intestinal homeostasis by altering gut physiology, and has been implicated in the pathophysiology of various GI disorders such as IBD, IBS and GI infection[94-97]. Using the Trichuris muris-infected AKR (susceptible to infection with generation of a Th1 response), BALB/c (resistant to infection, with generation of a Th2 response), STAT4-deficient (impaired in Th1 responses) and STAT6-deficient (impaired in Th2 responses) mice to explore the mechanism of the enterochromaffin (EC) cell and 5-HT responses in Th1/Th2-dominant environments, we found that the EC cell and 5-HT responses to the same infectious agent were influenced by Th1 or Th2 cytokine predominance[98].
Furthermore, we evaluated the 5-HT response and intestinal motility using T cell-induced enteropathy in Th1/Th2-dominant environments[99]. In BALB/c mice, carbachol-induced intestinal smooth muscle cell contraction was significantly increased at day 7 post anti-CD3 antibody injection, when the tissue damage returned to its normal histological appearance. We observed that 5-HT protein in the intestine was significantly increased at day 7. On the other hand, in AKR mice, carbachol-induced muscle cell contraction was significantly decreased and 5-HT protein in the intestine was also decreased at day 7. We showed, in this model, that Th1 and Th2 cytokines had opposing effects on intestinal muscle contraction via 5-HT signaling in the post-inflammation phase.
Th17: Several disorders that were originally considered to be Th1-mediated have been reclassified as Th17-mediated inflammation[100,101]. A recent study has shown that Th17 cells are increased during acute infection with T. spiralis, and that jejunal smooth muscle strips cultured with IL-17 show enhanced contractions, elicited by acetylcholine, in a concentration-dependent manner[102]. We found that IL-17 protein in the small intestine is upregulated in mice injected with an anti-CD3 antibody[103], and that IL-17 incubation with smooth muscle cells enhances carbachol-induced smooth muscle cell contraction (unpublished observations). IL-17 might be the key cytokine to alter GI muscle function.
As we have mentioned in this review, several cytokines are upregulated in GI diseases, and adaptive immune systems have a key role in altered muscle function of chronic GI diseases such as IBD and FGID.
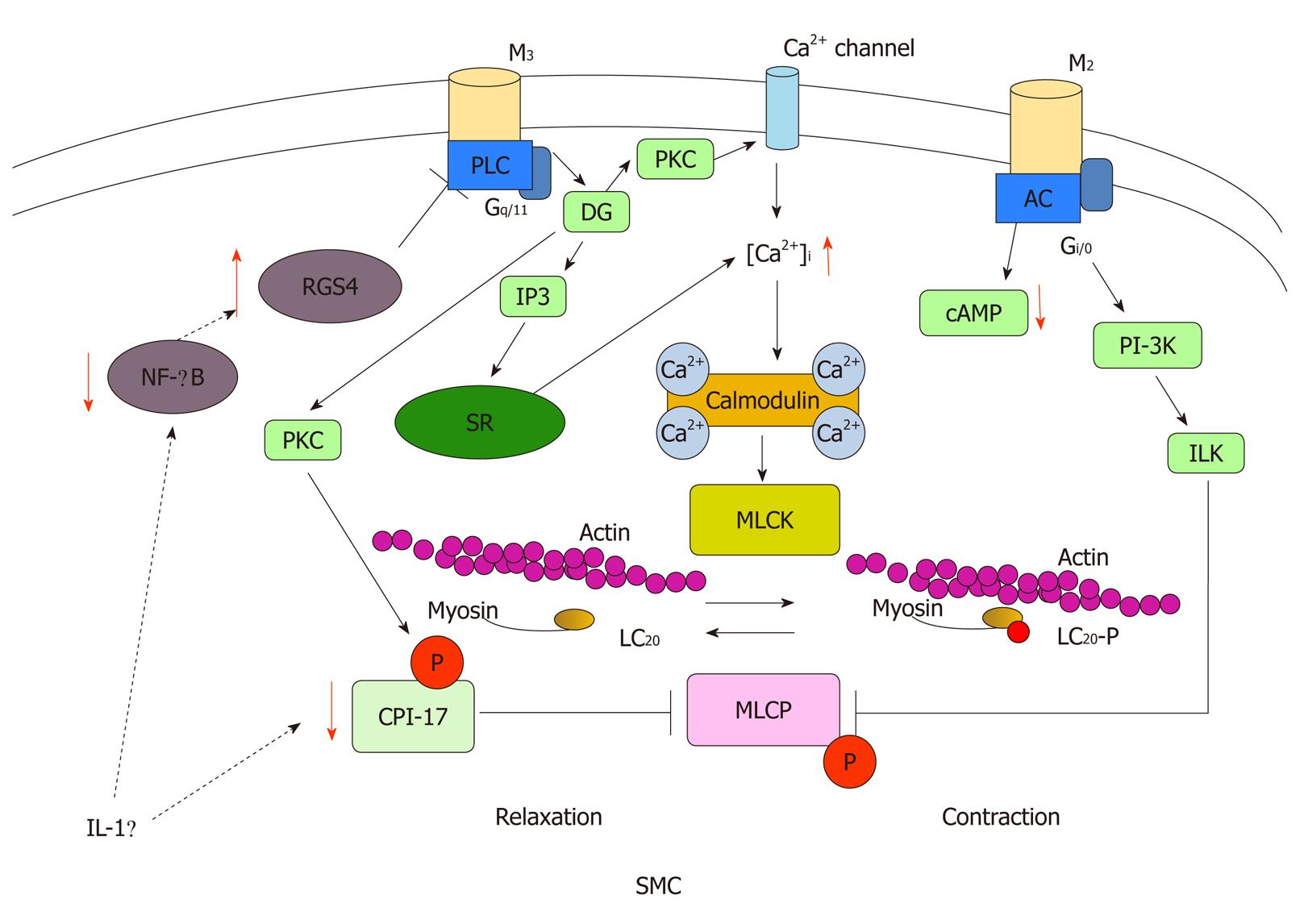
Motility disorder is a broad spectrum disturbance of GI physiology, including altered epithelial, smooth muscle, intestinal and enteric neural function and while immune activation may contribute, it plays only a limited role (Figure 2). GI motility depends on activation and coupling of muscarinic receptors at multiple sites including enteric neurons, ICCs, and smooth muscle. Cycles (slow waves) of membrane depolarization and repolarization originating in ICCs are transmitted to the smooth muscle cells. Among these, smooth muscle is the most important system because alteration in the contractile process occurs at the level of the GI smooth muscle. The depolarization of smooth muscle cells primarily reflects activation of voltage-gated Ca2+ channels, which results in Ca2+ entry from the extracellular space. Concurrent stimulation of rhythmic smooth muscle by excitatory neurotransmitters elicits further depolarization and Ca2+ entry, and activates intracellular signaling cascades that result in Ca2+ release from intracellular stores. GI smooth muscle expresses both M2 and M3 muscarinic receptors. The M3 receptors are coupled to Gq/11, which activates phospholipase C and produce inositol 1,4,5-triphosphate and diacylglycerol. These second messengers elicit the activation of PKC and trigger an increase in intracellular Ca2+ concentration ([Ca2+]i). On the other hand, M2 receptors are coupled to Gi/o, which regulates adenyl cyclase. Although the inhibition of adenyl cyclase is a classical effect of M2 receptor activation, other possible downstream signaling pathways which contribute to smooth muscle contraction have been proposed, including phosphoinositol 3-kinase and integrin-linked kinase[104]. Alternatively, coupling of M2 and M3 receptors is regulated by G protein receptor kinases and regulators of G protein signaling (RGS) proteins, which also play an important role in regulating smooth muscle contraction[105].
While increased [Ca2+]i is the paramount signal to initiate smooth muscle contraction, the contractile properties of smooth muscle cells are primarily governed by phosphorylation of the regulatory light chain (LC20) of myosin II[106,107]; this is in turn driven by the balance between myosin light chain kinase (MLCK) and smooth muscle myosin light chain phosphatase (MLCP). To initiate contraction, increases in [Ca2+]i activate MLCK, a Ca2+/calmodulin-dependent enzyme[108]. MLCK phosphorylates LC20 on Ser-19, which results in contraction of smooth muscle through increases in myosin ATPase activity and cross-bridge cycling. MLCP is responsible for the dephosphorylation of LC20, which results in relaxation of smooth muscle[109]. It is the balance between MLCK and MLCP activities that dictates the contractile activity of smooth muscle. Although MLCK is Ca2+/calmodulin dependent, MLCP functions independently of Ca2+/calmodulin and is mediated by the G protein-coupled process described above; it is regulated directly by phosphorylation of the myosin targeting subunit of MLCP (MYPT1)[109] and/or indirectly via phosphorylation of CPI-17[110]. Inhibition of MLCP, thus, results in greater LC20 phosophorylation and greater force development at a given [Ca2+]i.
It has been reported that IL-1β plays an important role in decreased GI smooth muscle contractility in Th1 cytokines-dominant colitis. It has been shown that IL-1β downregulates CPI-17 expression, which contributes to decreased GI smooth muscle contractility[40,41,84]. It has also been shown that IL-1β upregulates RGS4 expression by inhibiting NF-κB activation and RGS4 contributes to the inhibitory effect of IL-1β on the GI smooth muscle contraction[111,112]. Th2 cytokines may have opposing mechanisms to downregulate RGS4 expression. The important point is that it has yet to be determined whether the activated cytokines indicated above actually contribute to alteration of GI motility disorder in humans. However, several animal studies have shown that cytokines directly affect GI motility[84,87,89,102]. Further investigations should be undertaken.
Understanding the underlying immunological basis of GI disease by considering the time course of the disease, cytokine profile, and motor function may ultimately lead to new therapeutic strategies for GI functional and inflammatory disorders.
Peer reviewers: Zhao-Xiang Bian, Professor, School of Chinese Medicine, Hong Kong Baptist University, Hong Kong, China; Shi Liu, Professor, Union Hospital of Tongji Medical College, Department of Gastroenterology, Huazhong University of Science and Technology, 1277 Jie Fang Road, Wuhan 430022, Hubei Province, China
S- Editor Wu X L- Editor Hughes D E- Editor Zheng XM
| 1. | Bercík P, De Giorgio R, Blennerhassett P, Verdú EF, Barbara G, Collins SM. Immune-mediated neural dysfunction in a murine model of chronic Helicobacter pylori infection. Gastroenterology. 2002;123:1205-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Vallance BA, Croitoru K, Collins SM. T lymphocyte-dependent and -independent intestinal smooth muscle dysfunction in the T. spiralis-infected mouse. Am J Physiol. 1998;275:G1157-G1165. [PubMed] [Cited in This Article: ] |
| 3. | Myers BS, Dempsey DT, Yasar S, Martin JS, Parkman HP, Ryan JP. Acute experimental distal colitis alters colonic transit in rats. J Surg Res. 1997;69:107-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Krauter EM, Strong DS, Brooks EM, Linden DR, Sharkey KA, Mawe GM. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol Motil. 2007;19:990-1000. [PubMed] [Cited in This Article: ] |
| 5. | Radojevic N, McKay DM, Merger M, Vallance BA, Collins SM, Croitoru K. Characterization of enteric functional changes evoked by in vivo anti-CD3 T cell activation. Am J Physiol. 1999;276:R715-R723. [PubMed] [Cited in This Article: ] |
| 6. | Mizutani T, Akiho H, Khan WI, Murao H, Ogino H, Kanayama K, Nakamura K, Takayanagi R. Persistent gut motor dysfunction in a murine model of T-cell-induced enteropathy. Neurogastroenterol Motil. 2010;22:196-203, e65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Akiho H, Ihara E, Nakamura K. Low-grade inflammation plays a pivotal role in gastrointestinal dysfunction in irritable bowel syndrome. World J Gastrointest Pathophysiol. 2010;1:97-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 63] [Cited by in F6Publishing: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Di Lorenzo C. Pseudo-obstruction: current approaches. Gastroenterology. 1999;116:980-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 114] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 9. | Collins SM. Translating symptoms into mechanisms: functional GI disorders. Adv Physiol Educ. 2007;31:329-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Ma J, Zhang YG, Xia Y, Sun J. The inflammatory cytokine tumor necrosis factor modulates the expression of Salmonella typhimurium effector proteins. J Inflamm (Lond). 2010;7:42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Li Y, Reichenstein K, Ullrich R, Danner T, von Specht BU, Hahn HP. Effect of in situ expression of human interleukin-6 on antibody responses against Salmonella typhimurium antigens. FEMS Immunol Med Microbiol. 2003;37:135-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Lin CH, Hsieh CC, Chen SJ, Wu TC, Chung RL, Tang RB. The diagnostic value of serum interleukins 6 and 8 in children with acute gastroenteritis. J Pediatr Gastroenterol Nutr. 2006;43:25-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Li YP, Vegge CS, Brøndsted L, Madsen M, Ingmer H, Bang DD. Campylobacter jejuni induces an anti-inflammatory response in human intestinal epithelial cells through activation of phosphatidylinositol 3-kinase/Akt pathway. Vet Microbiol. 2011;148:75-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Vermillion DL, Huizinga JD, Riddell RH, Collins SM. Altered small intestinal smooth muscle function in Crohn's disease. Gastroenterology. 1993;104:1692-1699. [PubMed] [Cited in This Article: ] |
| 15. | Snape WJ, Williams R, Hyman PE. Defect in colonic smooth muscle contraction in patients with ulcerative colitis. Am J Physiol. 1991;261:G987-G991. [PubMed] [Cited in This Article: ] |
| 16. | Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet. 2008;40:955-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2041] [Cited by in F6Publishing: 2018] [Article Influence: 126.1] [Reference Citation Analysis (0)] |
| 17. | Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461-1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2329] [Cited by in F6Publishing: 2249] [Article Influence: 124.9] [Reference Citation Analysis (0)] |
| 18. | Anderson CA, Massey DC, Barrett JC, Prescott NJ, Tremelling M, Fisher SA, Gwilliam R, Jacob J, Nimmo ER, Drummond H. Investigation of Crohn's disease risk loci in ulcerative colitis further defines their molecular relationship. Gastroenterology. 2009;136:523-9.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Franke A, Balschun T, Karlsen TH, Hedderich J, May S, Lu T, Schuldt D, Nikolaus S, Rosenstiel P, Krawczak M. Replication of signals from recent studies of Crohn's disease identifies previously unknown disease loci for ulcerative colitis. Nat Genet. 2008;40:713-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 20. | Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut. 2009;58:1152-1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 467] [Cited by in F6Publishing: 498] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 21. | Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3754] [Cited by in F6Publishing: 3697] [Article Influence: 246.5] [Reference Citation Analysis (0)] |
| 22. | Annese V, Bassotti G, Napolitano G, Usai P, Andriulli A, Vantrappen G. Gastrointestinal motility disorders in patients with inactive Crohn's disease. Scand J Gastroenterol. 1997;32:1107-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 100] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Götze H, Ptok A. Orocaecal transit time in patients with Crohn disease. Eur J Pediatr. 1993;152:193-196. [PubMed] [Cited in This Article: ] |
| 24. | Hyams JS, Fitzgerald JE, Wyzga N, Treem WR, Justinich CJ, Kreutzer DL. Characterization of circulating interleukin-1 receptor antagonist expression in children with inflammatory bowel disease. Dig Dis Sci. 1994;39:1893-1899. [PubMed] [Cited in This Article: ] |
| 25. | Kohno N, Nomura M, Okamoto H, Kaji M, Ito S. The use of electrogastrography and external ultrasonography to evaluate gastric motility in Crohn's disease. J Med Invest. 2006;53:277-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Akiho H, Lovato P, Deng Y, Ceponis PJ, Blennerhassett P, Collins SM. Interleukin-4- and -13-induced hypercontractility of human intestinal muscle cells-implication for motility changes in Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2005;288:G609-G615. [PubMed] [Cited in This Article: ] |
| 27. | Akiho H, Blennerhassett P, Deng Y, Collins SM. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G226-G232. [PubMed] [Cited in This Article: ] |
| 28. | Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550-564. [PubMed] [Cited in This Article: ] |
| 29. | Fuss IJ, Strober W, Dale JK, Fritz S, Pearlstein GR, Puck JM, Lenardo MJ, Straus SE. Characteristic T helper 2 T cell cytokine abnormalities in autoimmune lymphoproliferative syndrome, a syndrome marked by defective apoptosis and humoral autoimmunity. J Immunol. 1997;158:1912-1918. [PubMed] [Cited in This Article: ] |
| 30. | Olsen T, Goll R, Cui G, Husebekk A, Vonen B, Birketvedt GS, Florholmen J. Tissue levels of tumor necrosis factor-alpha correlates with grade of inflammation in untreated ulcerative colitis. Scand J Gastroenterol. 2007;42:1312-1320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V, Achkar JP, Goyette P, Scott R, Xu W. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet. 2009;41:216-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 323] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 32. | Kobayashi T, Okamoto S, Hisamatsu T, Kamada N, Chinen H, Saito R, Kitazume MT, Nakazawa A, Sugita A, Koganei K. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut. 2008;57:1682-1689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 424] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 33. | Dambacher J, Beigel F, Zitzmann K, De Toni EN, Göke B, Diepolder HM, Auernhammer CJ, Brand S. The role of the novel Th17 cytokine IL-26 in intestinal inflammation. Gut. 2009;58:1207-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 34. | Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827-G838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 429] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 35. | Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 379] [Cited by in F6Publishing: 391] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 36. | Vrees MD, Pricolo VE, Potenti FM, Cao W. Abnormal motility in patients with ulcerative colitis: the role of inflammatory cytokines. Arch Surg. 2002;137:439-45; discussion 445-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Reddy SN, Bazzocchi G, Chan S, Akashi K, Villanueva-Meyer J, Yanni G, Mena I, Snape WJ. Colonic motility and transit in health and ulcerative colitis. Gastroenterology. 1991;101:1289-1297. [PubMed] [Cited in This Article: ] |
| 38. | Schoen RE, Wald A. Colonic motility in ulcerative colitis: muscling in on a mucosal disease? Am J Gastroenterol. 1992;87:1674-1675. [PubMed] [Cited in This Article: ] |
| 39. | Terry PD, Villinger F, Bubenik GA, Sitaraman SV. Melatonin and ulcerative colitis: evidence, biological mechanisms, and future research. Inflamm Bowel Dis. 2009;15:134-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Ohama T, Hori M, Fujisawa M, Kiyosue M, Hashimoto M, Ikenoue Y, Jinno Y, Miwa H, Matsumoto T, Murata T. Downregulation of CPI-17 contributes to dysfunctional motility in chronic intestinal inflammation model mice and ulcerative colitis patients. J Gastroenterol. 2008;43:858-865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Ohama T, Hori M, Sato K, Ozaki H, Karaki H. Chronic treatment with interleukin-1beta attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J Biol Chem. 2003;278:48794-48804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Barquist E, Zinner M, Rivier J, Taché Y. Abdominal surgery-induced delayed gastric emptying in rats: role of CRF and sensory neurons. Am J Physiol. 1992;262:G616-G620. [PubMed] [Cited in This Article: ] |
| 43. | Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg. 1998;228:652-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 391] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 44. | Kreiss C, Birder LA, Kiss S, VanBibber MM, Bauer AJ. COX-2 dependent inflammation increases spinal Fos expression during rodent postoperative ileus. Gut. 2003;52:527-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Kreiss C, Toegel S, Bauer AJ. Alpha2-adrenergic regulation of NO production alters postoperative intestinal smooth muscle dysfunction in rodents. Am J Physiol Gastrointest Liver Physiol. 2004;287:G658-G666. [PubMed] [Cited in This Article: ] |
| 46. | Wehner S, Schwarz NT, Hundsdoerfer R, Hierholzer C, Tweardy DJ, Billiar TR, Bauer AJ, Kalff JC. Induction of IL-6 within the rodent intestinal muscularis after intestinal surgical stress. Surgery. 2005;137:436-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Frasko R, Maruna P, Gurlich R, Trca S. Transcutaneous electrogastrography in patients with ileus. Relations to interleukin-1beta, interleukin-6, procalcitonin and C-reactive protein. Eur Surg Res. 2008;41:197-202. [PubMed] [Cited in This Article: ] |
| 48. | Moore BA, Albers KM, Davis BM, Grandis JR, Tögel S, Bauer AJ. Altered inflammatory gene expression underlies increased susceptibility to murine postoperative ileus with advancing age. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1650-G1659. [PubMed] [Cited in This Article: ] |
| 49. | Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol Motil. 2004;16 Suppl 2:54-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 242] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 50. | Kalff JC, Schraut WH, Billiar TR, Simmons RL, Bauer AJ. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology. 2000;118:316-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 217] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 51. | Schwarz NT, Kalff JC, Türler A, Engel BM, Watkins SC, Billiar TR, Bauer AJ. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology. 2001;121:1354-1371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 192] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 52. | Mittal RK, Kassab G, Puckett JL, Liu J. Hypertrophy of the muscularis propria of the lower esophageal sphincter and the body of the esophagus in patients with primary motility disorders of the esophagus. Am J Gastroenterol. 2003;98:1705-1712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Robertson CS, Martin BA, Atkinson M. Varicella-zoster virus DNA in the oesophageal myenteric plexus in achalasia. Gut. 1993;34:299-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 110] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Facco M, Brun P, Baesso I, Costantini M, Rizzetto C, Berto A, Baldan N, Palù G, Semenzato G, Castagliuolo I. T cells in the myenteric plexus of achalasia patients show a skewed TCR repertoire and react to HSV-1 antigens. Am J Gastroenterol. 2008;103:1598-1609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Kilic A, Owens SR, Pennathur A, Luketich JD, Landreneau RJ, Schuchert MJ. An increased proportion of inflammatory cells express tumor necrosis factor alpha in idiopathic achalasia of the esophagus. Dis Esophagus. 2009;22:382-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Yan BM, Shaffer EA. Primary eosinophilic disorders of the gastrointestinal tract. Gut. 2009;58:721-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 57. | Pardi DS, Kelly CP. Microscopic colitis. Gastroenterology. 2011;140:1155-1165. [PubMed] [Cited in This Article: ] |
| 58. | Tagkalidis PP, Gibson PR, Bhathal PS. Microscopic colitis demonstrates a T helper cell type 1 mucosal cytokine profile. J Clin Pathol. 2007;60:382-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 59. | Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731-1743. [PubMed] [Cited in This Article: ] |
| 60. | Jabri B, Sollid LM. Mechanisms of disease: immunopathogenesis of celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:516-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 61. | Spiller R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol Motil. 2007;19 Suppl 2:25-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 62. | Challacombe DN, Dawkins PD, Baker P. Increased tissue concentrations of 5-hydroxytryptamine in the duodenal mucosa of patients with coeliac disease. Gut. 1977;18:882-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Sjölund K, Nobin A. Increased levels of plasma 5-hydroxytryptamine in patients with coeliac disease. Scand J Gastroenterol. 1985;20:304-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Challacombe DN, Brown GA, Black SC, Storrie MH. Increased excretion of 5-hydroxyindoleacetic acid in urine of children with untreated coeliac disease. Arch Dis Child. 1972;47:442-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Monteleone I, Monteleone G, Del Vecchio Blanco G, Vavassori P, Cucchiara S, MacDonald TT, Pallone F. Regulation of the T helper cell type 1 transcription factor T-bet in coeliac disease mucosa. Gut. 2004;53:1090-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Fernández S, Molina IJ, Romero P, González R, Peña J, Sánchez F, Reynoso FR, Pérez-Navero JL, Estevez O, Ortega C. Characterization of gliadin-specific Th17 cells from the mucosa of celiac disease patients. Am J Gastroenterol. 2011;106:528-538. [PubMed] [Cited in This Article: ] |
| 67. | Coulie B, Camilleri M. Intestinal pseudo-obstruction. Annu Rev Med. 1999;50:37-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Selgrad M, De Giorgio R, Fini L, Cogliandro RF, Williams S, Stanghellini V, Barbara G, Tonini M, Corinaldesi R, Genta RM. JC virus infects the enteric glia of patients with chronic idiopathic intestinal pseudo-obstruction. Gut. 2009;58:25-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Sanders KM, Ordög T, Ward SM. Physiology and pathophysiology of the interstitial cells of Cajal: from bench to bedside. IV. Genetic and animal models of GI motility disorders caused by loss of interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2002;282:G747-G756. [PubMed] [Cited in This Article: ] |
| 70. | Talley NJ, Stanghellini V, Heading RC, Koch KL, Malagelada JR, Tytgat GN. Functional gastroduodenal disorders. Gut. 1999;45 Suppl 2:II37-II42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 445] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 71. | Kindt S, Van Oudenhove L, Broekaert D, Kasran A, Ceuppens JL, Bossuyt X, Fischler B, Tack J. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:389-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 72. | Arisawa T, Tahara T, Shibata T, Nagasaka M, Nakamura M, Kamiya Y, Fujita H, Yoshioka D, Arima Y, Okubo M. Genetic polymorphisms of molecules associated with inflammation and immune response in Japanese subjects with functional dyspepsia. Int J Mol Med. 2007;20:717-723. [PubMed] [Cited in This Article: ] |
| 73. | Futagami S, Shindo T, Kawagoe T, Horie A, Shimpuku M, Gudis K, Iwakiri K, Itoh T, Sakamoto C. Migration of eosinophils and CCR2-/CD68-double positive cells into the duodenal mucosa of patients with postinfectious functional dyspepsia. Am J Gastroenterol. 2010;105:1835-1842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 74. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3413] [Cited by in F6Publishing: 3325] [Article Influence: 184.7] [Reference Citation Analysis (1)] |
| 75. | Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778-1783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 563] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 76. | Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 345] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 77. | Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 805] [Cited by in F6Publishing: 806] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 78. | Ohman L, Isaksson S, Lindmark AC, Posserud I, Stotzer PO, Strid H, Sjövall H, Simrén M. T-cell activation in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:1205-1212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 79. | Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 452] [Cited by in F6Publishing: 478] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 80. | Dinan TG, Quigley EM, Ahmed SM, Scully P, O'Brien S, O'Mahony L, O'Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 448] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 81. | Scully P, McKernan DP, Keohane J, Groeger D, Shanahan F, Dinan TG, Quigley EM. Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am J Gastroenterol. 2010;105:2235-2243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 82. | Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 821] [Cited by in F6Publishing: 929] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 83. | Galeazzi F, Haapala EM, van Rooijen N, Vallance BA, Collins SM. Inflammation-induced impairment of enteric nerve function in nematode-infected mice is macrophage dependent. Am J Physiol Gastrointest Liver Physiol. 2000;278:G259-G265. [PubMed] [Cited in This Article: ] |
| 84. | Ohama T, Hori M, Momotani E, Iwakura Y, Guo F, Kishi H, Kobayashi S, Ozaki H. Intestinal inflammation downregulates smooth muscle CPI-17 through induction of TNF-alpha and causes motility disorders. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1429-G1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 85. | Kinoshita K, Sato K, Hori M, Ozaki H, Karaki H. Decrease in activity of smooth muscle L-type Ca2+ channels and its reversal by NF-kappaB inhibitors in Crohn's colitis model. Am J Physiol Gastrointest Liver Physiol. 2003;285:G483-G493. [PubMed] [Cited in This Article: ] |
| 86. | Schwarz NT, Kalff JC, Türler A, Speidel N, Grandis JR, Billiar TR, Bauer AJ. Selective jejunal manipulation causes postoperative pan-enteric inflammation and dysmotility. Gastroenterology. 2004;126:159-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 87. | Akiho H, Khan WI, Al-Kaabi A, Blennerhassett P, Deng Y, Collins SM. Cytokine modulation of muscarinic receptors in the murine intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G250-G255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 88. | Wells RW, Blennerhassett MG. Persistent and selective effects of inflammation on smooth muscle cell contractility in rat colitis. Pflugers Arch. 2004;448:515-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Akiho H, Deng Y, Blennerhassett P, Kanbayashi H, Collins SM. Mechanisms underlying the maintenance of muscle hypercontractility in a model of postinfective gut dysfunction. Gastroenterology. 2005;129:131-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 90. | Khan WI, Vallance BA, Blennerhassett PA, Deng Y, Verdu EF, Matthaei KI, Collins SM. Critical role for signal transducer and activator of transcription factor 6 in mediating intestinal muscle hypercontractility and worm expulsion in Trichinella spiralis-infected mice. Infect Immun. 2001;69:838-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 91. | Zhao A, Urban JF, Anthony RM, Sun R, Stiltz J, van Rooijen N, Wynn TA, Gause WC, Shea-Donohue T. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology. 2008;135:217-225.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 92. | Zhao A, McDermott J, Urban JF, Gause W, Madden KB, Yeung KA, Morris SC, Finkelman FD, Shea-Donohue T. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003;171:948-954. [PubMed] [Cited in This Article: ] |
| 93. | Ihara E, Beck PL, Chappellaz M, Wong J, Medlicott SA, MacDonald JA. Mitogen-activated protein kinase pathways contribute to hypercontractility and increased Ca2+ sensitization in murine experimental colitis. Mol Pharmacol. 2009;75:1031-1041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Camilleri M, Atanasova E, Carlson PJ, Ahmad U, Kim HJ, Viramontes BE, McKinzie S, Urrutia R. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 213] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 95. | Crowell MD, Shetzline MA, Moses PL, Mawe GM, Talley NJ. Enterochromaffin cells and 5-HT signaling in the pathophysiology of disorders of gastrointestinal function. Curr Opin Investig Drugs. 2004;5:55-60. [PubMed] [Cited in This Article: ] |
| 96. | Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 554] [Cited by in F6Publishing: 555] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 97. | Garvin B, Wiley JW. The role of serotonin in irritable bowel syndrome: implications for management. Curr Gastroenterol Rep. 2008;10:363-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Motomura Y, Ghia JE, Wang H, Akiho H, El-Sharkawy RT, Collins M, Wan Y, McLaughlin JT, Khan WI. Enterochromaffin cell and 5-hydroxytryptamine responses to the same infectious agent differ in Th1 and Th2 dominant environments. Gut. 2008;57:475-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 99. | Murao H, Akiho H, Mizutani T, Yamada M, Tokunaga N, Aso A, Ogino H, Kanayama K, Sumida Y, Iboshi Y. Reciprocal modulation of smooth muscle cell contractility in Th1 and Th2 dominant environments using murine model of early post inflammatory gut dysfunction. (Abstract). DDW. 2009;T1778. [Cited in This Article: ] |
| 100. | Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566-573. [PubMed] [Cited in This Article: ] |
| 101. | Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173-6177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 102. | Fu Y, Wang W, Tong J, Pan Q, Long Y, Qian W, Hou X. Th17: a new participant in gut dysfunction in mice infected with Trichinella spiralis. Mediators Inflamm. 2009;2009:517052. [PubMed] [Cited in This Article: ] |
| 103. | Akiho H, Nakamura K. Daikenchuto ameliorates muscle hypercontractility in a murine T-cell-mediated persistent gut motor dysfunction model. Digestion. 2011;83:173-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 104. | Huang J, Mahavadi S, Sriwai W, Hu W, Murthy KS. Gi-coupled receptors mediate phosphorylation of CPI-17 and MLC20 via preferential activation of the PI3K/ILK pathway. Biochem J. 2006;396:193-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 105. | Gerthoffer WT. Signal-transduction pathways that regulate visceral smooth muscle function. III. Coupling of muscarinic receptors to signaling kinases and effector proteins in gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2005;288:G849-G853. [PubMed] [Cited in This Article: ] |
| 106. | Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1453] [Cited by in F6Publishing: 1424] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 107. | Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325-1358. [PubMed] [Cited in This Article: ] |
| 108. | Taylor DA, Stull JT. Calcium dependence of myosin light chain phosphorylation in smooth muscle cells. J Biol Chem. 1988;263:14456-14462. [PubMed] [Cited in This Article: ] |
| 109. | Hartshorne DJ, Ito M, Erdödi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J Biol Chem. 2004;279:37211-37214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 110. | Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J Biochem. 1995;118:1104-1107. [PubMed] [Cited in This Article: ] |
| 111. | Hu W, Mahavadi S, Li F, Murthy KS. Upregulation of RGS4 and downregulation of CPI-17 mediate inhibition of colonic muscle contraction by interleukin-1beta. Am J Physiol Cell Physiol. 2007;293:C1991-C2000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 112. | Hu W, Li F, Mahavadi S, Murthy KS. Upregulation of RGS4 expression by IL-1beta in colonic smooth muscle is enhanced by ERK1/2 and p38 MAPK and inhibited by the PI3K/Akt/GSK3beta pathway. Am J Physiol Cell Physiol. 2009;296:C1310-C1320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |