Copyright
©The Author(s) 2023.
World J Gastrointest Pathophysiol. Aug 24, 2023; 14(4): 71-85
Published online Aug 24, 2023. doi: 10.4291/wjgp.v14.i4.71
Published online Aug 24, 2023. doi: 10.4291/wjgp.v14.i4.71
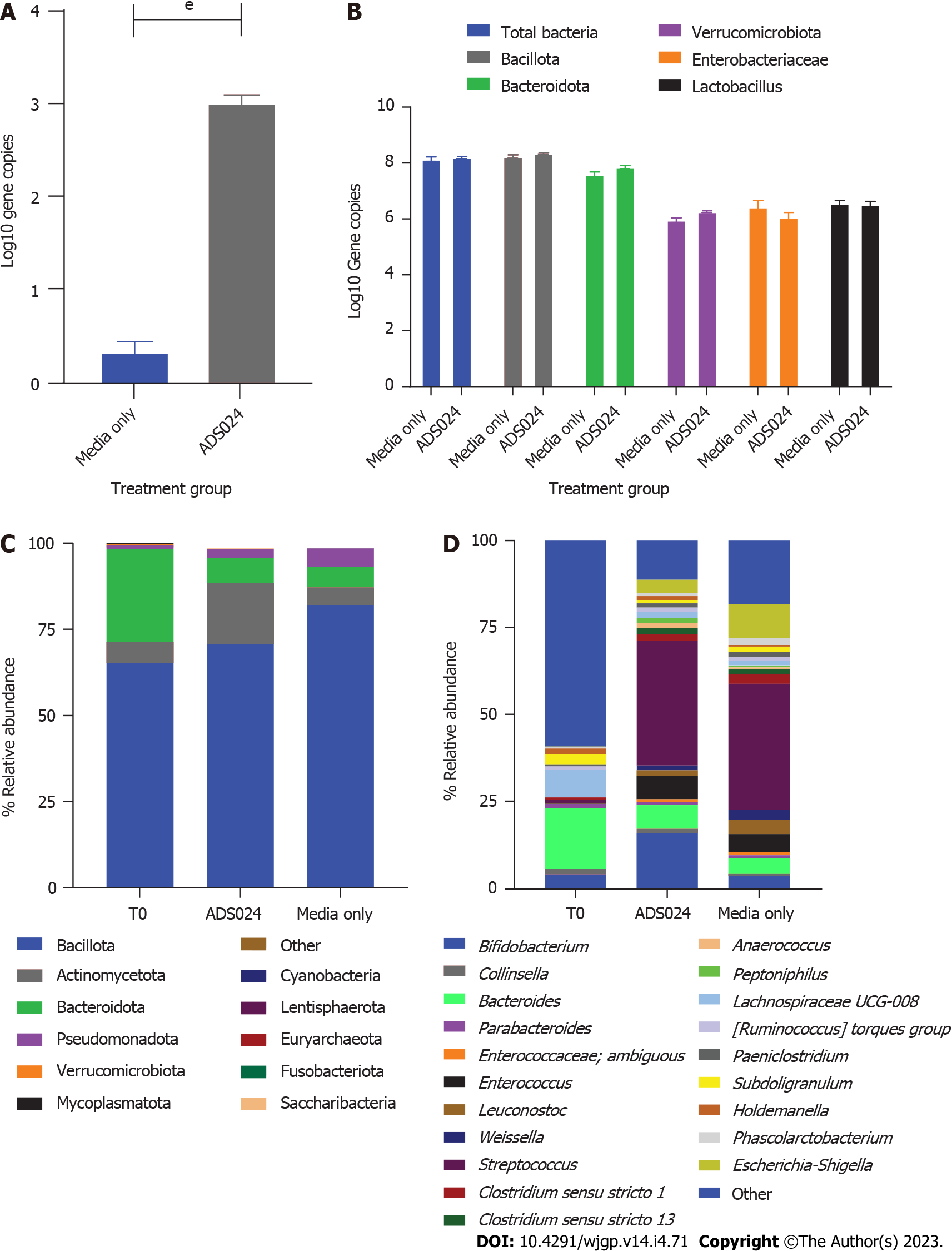
Figure 3 Distal colon model.
A: Comparison of the wells inoculated with ADS024 vs media alone was done via quantitative polymerase chain reaction (qPCR) using specific primers to detect ADS024 as described in Materials and Methods; B: qPCR was performed to study the antimicrobial activity of ADS024 on 5 different phyla of the human colonic microbiota; C: MiSeq compositional sequencing analysis was performed to compare the impact of ADS024 on the gut microbiota at the phylum level with that of media alone; D: MiSeq was used to determine the ADS024 effect after 24 h of treatment at the genus level. eP ≤ 0.001.
- Citation: Murphy CK, O’Donnell MM, Hegarty JW, Schulz S, Hill C, Ross RP, Rea MC, Farquhar R, Chesnel L. Novel, non-colonizing, single-strain live biotherapeutic product ADS024 protects against Clostridioides difficile infection challenge in vivo. World J Gastrointest Pathophysiol 2023; 14(4): 71-85
- URL: https://www.wjgnet.com/2150-5330/full/v14/i4/71.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v14.i4.71