INTRODUCTION
Congenital abnormalities of the kidney and urinary tract occur in 3-6 per 1000 live births and is the leading cause of end-stage renal disease (ESRD) in children and also cause subsequent renal problems in adulthood like stone formation, infection, hypertension, and renal failure[1]. The North American Pediatric Renal Trials and Collaborative Studies report indicated that 30% to 50% of cases of ESRD are related to congenital anomalies of the kidney and the urinary tract[2]. Therefore, it is crucial to have early diagnosis and management, whether medical or surgical, to minimize renal damage and to avoid or delay end-stage renal damage. Imaging helps in the early diagnosis, follow-up, surgical planning, detection of complications and associated renal and extra renal malformations. This review summarizes the various congenital anomalies of kidneys and upper urinary tract, their embryological basis, clinical presentation, imaging features and complications.
RELEVANT EMBRYOLOGY
The kidneys develop from three structures which succeed each other: The pronephros and the mesonephric and metanephric ducts. Pronephros is the most immature form of kidney and develops from intermediate mesoderm towards the end of 3rd week of gestation. It is non-functional in human beings and regresses by 4th week leaving no adult lineage and replaced by mesonephros which later forms the portion of the male and female genital tracts. The kidneys develop during the 4th week of gestation by the union of the ureteric bud with the metanephric mass of intermediate mesoderm at the level of the first or second sacral segment. The kidneys initially lie close to each other with the hila directed anteriorly in the pelvis. During 4th to 8th weeks of gestation both kidneys gradually ascend to the lumbar region moving further apart from each other and rotate medially almost 90 degrees with the hila now facing anteromedially[3,4].
CLASSIFICATION
Congenital anomalies of the kidneys can be classified on embryological basis in to abnormalities in the renal parenchymal development, aberrant embryonic migration and abnormalities of the collecting system[5]. Renal parenchymal abnormalities include multi cystic dysplastic kidneys (MCDK), renal hypoplasia, number (agenesis or supernumary), shape and cystic renal diseases. Aberrant embryonic migration encompasses abnormal location and fusion anomalies. Collecting system abnormalities include duplex kidneys and Pelvi ureteric junction obstruction (PUJO). We have included only upper urinary system anomalies in this review.
CLINICAL PRESENTATION AND IMAGING APPROACH
Clinical features vary widely depending on the type, severity and laterality of renal anomaly. Many of them like hypoplasia, ectopic kidneys, and anomalies in shape can be asymptomatic and detected incidentally in adults. Others like renal agenesis, MCDK, bilateral PUJO and cystic renal diseases can present early either antenatally with oligohydramnios or later with urinary tract infection, hypertension, proteinuria, renal impairment, abdominal mass, hematuria or stones[6].
Antenatal screening ultrasonography (US) allows demonstration of fetal kidneys and urinary bladder from early second trimester and enables to detect a number of major congenital anomalies of the urinary system. The ultrasound examination of the normal urinary tract consists of the assessment of the presence, location and size of both kidneys and the evaluation of their structure and echogenicity. Evaluation of fetal bladder, external genitalia and the amount of amniotic fluid compliments the examination. Abnormalities detected in antenatal US include hydronephrosis, hydroureter, thickened bladder, cystic kidney, small or dysplastic kidney and absent kidney. Antenatal detection of renal abnormalities warrants postnatal physical examination and postnatal renal ultrasound soon after birth and again at 4-6 wk of age[5,7].
Cross sectional imaging include computed tomography (CT) and magnetic resonance imaging (MRI) help in resolving the complex anatomy, ectopic kidneys not detected on US, duplex collecting system, preoperative evaluation in PUJO and to look for complications like pyelonephritis, renal stones, and malignancies. Non contrast CT can detect renal stones and nephrocalcinosis. CT urography is useful in duplex system, their complex course, distal opening and associated other genitourinary malformations. MRI has the advantages of lack of ionizing radiation, better soft tissue contrast and detection of collecting system abnormalities even without contrast[8]. Disadvantage is requirement of sedation in small children, cost and availability[3,9,10].
RENAL PARENCHYMAL MALFORMATION
Anomalies in number
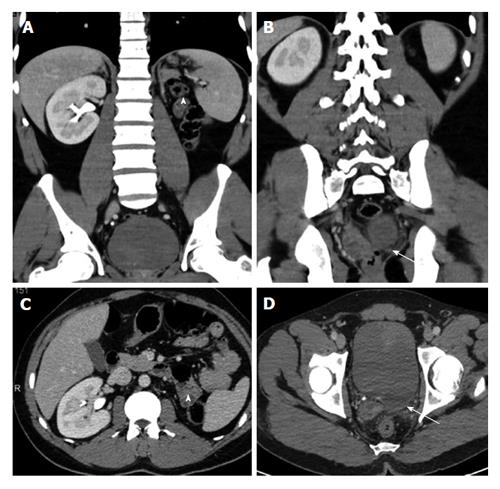
Renal agenesis: Complete absence of one or both kidneys indicates renal agenesis. It thought to result from a lack of induction of the metanephric blastema by the ureteral bud. Bilateral agenesis is incompatible with life and is associated with pulmonary hypoplasia and limb defects. Unilateral renal agenesis is not uncommon, seen in 1/1300 pregnancies[11]. It is often asymptomatic and compensatory hypertrophy in other kidney may cause glomerulosclerosis in adults. Unilateral renal agenesis is often incidentally detected in adults in US or CT performed for other reasons. Associated abnormalities can be seen like ipsilateral seminal vesicle cyst, ipsilateral absence of seminal vesicle or mullerian abnormalities[12,13] (Figures 1 and 2).
Figure 1 A 35-year-old man with left renal agenesis.
Coronal (A and B) and axial (C and D) contrast enhanced CT shows absent left kidney (arrowhead) and absent left seminal vesicle (arrow). CT: Computed tomography.
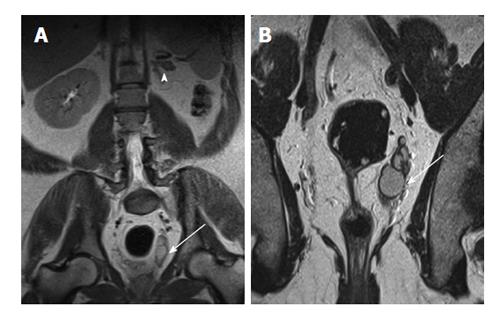
Figure 2 A 40-year-old man with left renal agenesis.
Coronal T2W magnetic resonance imaging abdomen (A) and pelvis (B) shows absent left kidney (arrowhead) and left seminal vesicle cyst (arrow).
Supernumary kidneys: A supernumerary kidney is an uncommon urogenital anomaly with less than 100 cases reported in the literature. In this one or more accessory kidneys are seen often on the left and caudal to the native kidney. Mostly the accessory kidney is smaller in size with reduced function. It can be detected on US, CT, MRI and Nuclear Medicine studies like dimercaptosuccinic acid (DMSA) and diethylene triamine pentaacetic acid (DTPA) scans. US is useful in the morphological characterisation while the rest aid in functional assessment. Supernumary kidneys commonly have associated various urogenital and non urogenital anomalies[14,15].
Anomalies in shape
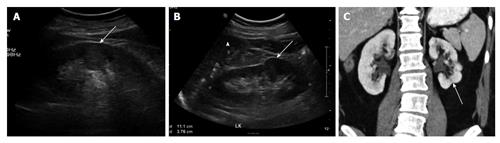
Persistent fetal lobulations: Persistent fetal lobulations is a normal variant seen in adult kidneys due to incomplete fusion of the developing renal lobules and could be mistaken for renal scarring. However it is seen as smooth indentation of the renal outline in-between the pyramids on ultrasonogram (USG), CT OR MRI as compared to renal scarring where indentation is not smooth, asymmetrical and it overlies the renal pyramids[16] (Figure 3).
Figure 3 Persistent fetal lobulations.
A: USG shows pseudomass from hypertrophied column of Bertin (arrow); B: USG shows Dromedary hump (arrow) from splenic impression (arrowhead); C: Coronal CT shows small LK with persistent fetal lobulations (arrow) inbetween the pyramids. CT: Computed tomography; USG: Ultrasonogram; LK: Left kidney.
Hypertrophied column of bertini: It is the extension of renal cortical tissue which separates the pyramids towards the central parenchyma. It is important as it can be mistaken for a renal mass on US. It is usually located in the mid portion of the left kidney. Key to correct identification is the fact that it is in continuity with, and have signature of normal renal parenchyma regardless of the type of imaging modality, CT or MRI[17,18] (Figures 3-5).
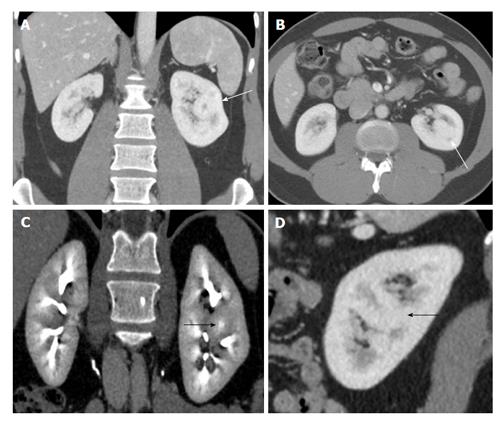
Figure 4 A 50-year-old woman with suspicious solid mass in left kidney on ultrasonogram.
Coronal (A and C), axial (B) and sagittal (D) computed tomography showing hypertrophied column of Bertin in interpolar region of LK (arrows) showing density and enhancement pattern similar to adjacent normal renal cortex. LK: Left kidney.
Figure 5 A 60-year-old man with suspicious mass in the left kidney on ultrasonogram.
Coronal T2W (A) and contrast enhanced T1W magnetic resonance imaging (B) shows hypertrophied column of Bertin in interpolar region of LK (arrows). LK: Left kidney.
Dromedary hump: Normal variant of renal contour appearing as focal contour bulge, caused by the splenic impression onto the superolateral left kidney. It can mimic a renal mass and the key to diagnosis is it is identical to renal parenchyma in density/signal intensity on both precontrast and contrast sequences. Another clue is calyces underlying the hump extend further laterally into the hump than the other calyces[16] (Figure 3).
Renal hilar lip: A renal hilar lip is an infolding of the cortex at the level of the renal sinus giving a pseudo mass appearance. Again cortical signature and careful evaluation of contiguous images on CT or MRI help in resolving the confusion[19].
Junctional parenchymal defect: It is a normal variant seen as a triangular echogenic area due to the extension of sinus fat into the cortex. It results from embryonic fusion of renunculi. It can be distinguished from small angiomyolipoma (AML) or scar by its continuity with renal sinus fat and its typical location in interpolar region[16,17].
Renal hypoplasia
Renal hypoplasia refers to congenital small kidney with lesser number of calices and papilla (< 6) due to incomplete renal development. It is often unilateral and can have normal or mildly reduced function. It can be global or segmental. Global hypoplasia needs to be differentiated from atrophic kidneys due to pyelonephritis or chronic vascular diseases in adults. Atrophic kidneys from pyelonephritis usually show irregular outline from scarring with focal dilatation/clubbing of calices in contrast to smooth outline and non-dilated calices in global hypoplasia. Small smooth kidney from chronic vascular diseases are difficult to differentiate from hypoplasia[4]. Segmental renal hypoplasia also known as Ask-Upmark kidney is a cause of secondary hypertension in young adults. Imaging reveals small nonfunctioning kidney or focal hypoplasia of upper or lower pole and final diagnosis is made on characteristic histological features[4,5,20] (Figures 6 and 7).
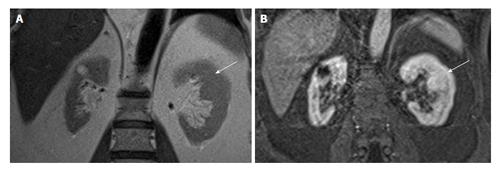
Figure 6 A 35-year-old man presented with right flank pain and incidental left renal hypoplasia.
Coronal (A) and axial (B) non-contrast computed tomography shows uniformly small left kidney with no scarring (arrow). Compensatory hypertrophy of right kidney seen with tiny calculi.
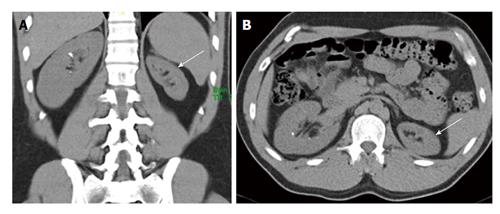
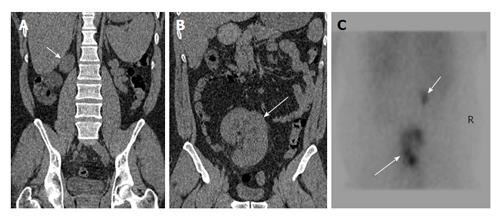
Figure 7 A 45-year-old man presenting with recurrent lower abdominal pain.
Coronal non-contrast computed tomography (A and B) shows hypoplastic right kidney (short arrow) and ectopic left kidney (long arrow). Nuclear scan (C) show small poorly functioning right kidney (short arrow) and ectopic left kidney (long arrow).
Renal dysplasia
MCDK is a developmental disorder of the kidney, in which the normal renal parenchyma is replaced by multiple, non-communicating cysts of varying size with poorly identified echogenic parenchyma and atretic upper ureters. The incidence is about 1/4300 pregnancies[21]. USG (antenatal or postnatal) is diagnostic and should be differentiated from hydronephrosis which have communicating cysts[7]. Polycystic kidney disease presenting in adults shows multiple non communicating cysts similar to MCDK. However adult age of onset, bilateralism, enlarged kidney, intervening normal renal parenchyma and positive family history are helpful in differentiating from MCDK[22]. DMSA can be performed to establish non functioning kidney (Figure 8). In 25%-40% the contralateral kidney will also be abnormal, reflux being the most frequently associated anomaly and should be investigated with a voiding cysto urethrogram. The natural course in utero is variable and the final result is a non-functional kidney[23]. Bilateral multicystic kidney disease occurs in about 10%-20% of cases and is a lethal condition.
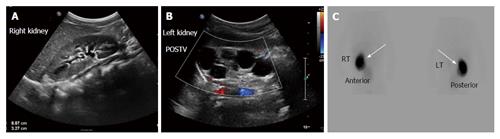
Figure 8 A 15-year-old girl with non-specific abdominal pain.
USG (A and B) shows normal right kidney and multiple non communicating cysts replacing left kidney. Nuclear scan (C) non functioning left kidney (arrows). USG: Ultrasonogram.
ABNORMAL MIGRATION
Congenital renal anomalies in the position and in the renal fusion are the result of impaired cephalic migration from the pelvis to the flank of the ureteric bud and metanephric blastema. This process of ascent begins in 5th week and ends at 9th week of gestation. It includes both ectopic location of kidneys and abnormal fusion of whole or part of the kidneys.
Ectopic kidneys
Renal ectopia is a congenital renal anomaly characterized by abnormal location of kidneys outside the flank region (L1-L3 vertebral levels). It can be simple ectopia where ectopic kidney is located on the same as its ureter or crossed ectopia when it is located on the opposite from its ureteric orifice. Both can be unilateral or bilateral. Simple renal ectopia is seen in 1:3000 and bilateral in 10% of cases[24]. The location, in order of frequency, may be pelvic, iliac, abdominal or chest. Ectopic kidney is usually smaller with varying degree of malrotation. The ureter has a length according to the location of the kidney and blood supply is from iliac or infrarenal abdominal aorta with typically multiple arteries. USG is diagnostic in most of the cases and cross sectional imaging or nuclear medicine studies are needed when USG visualized is suboptimal due to bowel gas, small kidneys or intrathoracic location[25]. Crossed renal ectopia is seen in 1:7000 and often incidentally detected. It can be fused in 85% where the ectopic kidney fuses with the orthotopic kidneys[9,26]. Commonest fusion pattern is fusion of lower pole of orthotopic kidney to the upper pole of crossed ectopic kidney. Other described patterns include sigmoid, L shaped, discoid and cake kidneys. USG shows absent kidney on one side and careful evaluation often reveals it on the opposite lumbar or iliac region. Often there is associated other genitourinary malformations and ectopic kidneys are complicated by stones, infection or hypertension from multiple anomalous arteries[3]. CT urography is helpful in confirming the diagnosis and demonstration of collecting system, arterial supply and complications. Ectopic kidneys need to be differentiated from renal transplants, nephroptosis, surgical repositioning of kidneys and nephrectomy (Figures 9-11).
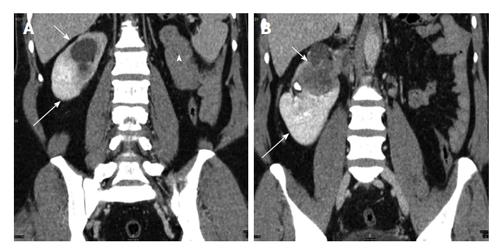
Figure 9 A 40-year-old man with long standing recurrent lower abdominal pain.
Coronal non-contrast computed tomography (A and B) shows ectopic left kidney in the pelvis with stag horn calculus causing hydronephrosis (arrows).
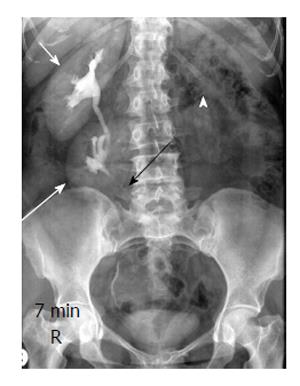
Figure 10 A 30-year-old man with crossed ectopia without fusion.
Intravenous urography shows crossed ectopia of left kidney (long arrow) below the normal right kidney (short arrow). Note ureter of ectopic left kidney crossing to left side (black arrow) and empty left renal fossa (arrowhead).
Figure 11 A 42-year-old man with crossed fused ectopia on the right side.
Coronal contrast enhanced computed tomography (A and B) shows empty left renal fossa (arrowhead) with crossed fused ectopic left kidney (long arrow) fused with the right kidney (short arrow) which shows infiltrating transitional cell carcinoma.
Horse shoe kidney
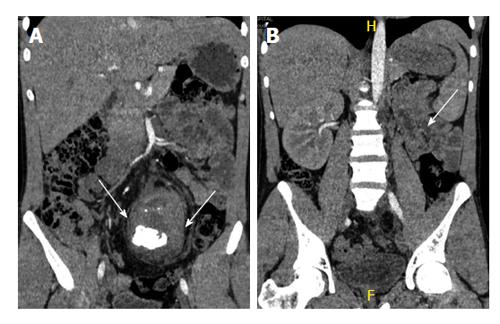
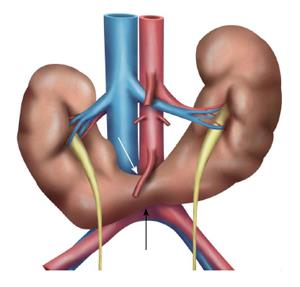
Horseshoe kidney is the most common congenital anomaly seen in 1 in 500 adults[27]. It is formed by fusion across the midline of two distinct functioning kidneys, one on each side of the midline. They are connected by an isthmus of functioning renal parenchyma or fibrous tissue. In the vast majority of cases the fusion is between the lower poles (90%). In the remainder the superior or both the superior or inferior poles are fused. It is due to normal ascent of kidneys impaired by inferior mesenteric artery which hooks over the isthmus resulting in a lower abdominal location and abnormal rotation, especially at the lower poles (Figure 12). The pelvis and ureters are anterior, ventrally crossing the isthmus[3]. Horse shoe kidney is prone to numerous complications due to its location and poor drainage like hydronephrosis, secondary to pelviureteric junction obstruction, renal calculus, infection, increased incidence of malignancy (Wilms tumour, transitional cell carcinoma, carcinoid and possibly renal cell carcinoma)[28] and increased susceptibility to trauma. On USG, clue to diagnosis is poor visualization of bilateral lower pole which should alert the sonographer to look for abnormal tissue anterior to aorta representing the isthmus. Lack of awareness could lead to overlooking the diagnosis with underestimation of renal length or mistaking the preaortic tissue for mass or lymphnode[17,29,30]. CT and MRI help in delineating the details of collecting system and arterial anatomy, differentiating functioning isthmus from fibrous tissue and for early detection of complications (Figures 13 and 14).
Figure 12 Schematic diagram showing the horse shoe kidneys due to the arrested migration of isthmus as the inferior mesenteric artery (white arrow) hooks over the isthmus (black arrow).
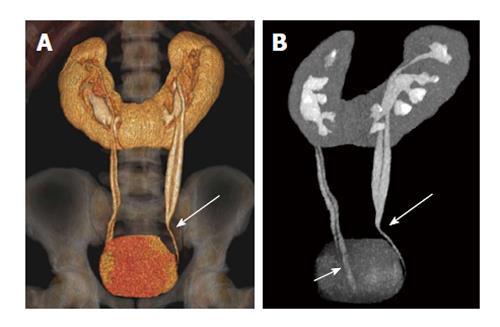
Figure 13 A 32-year-old woman with combined horse shoe kidney and duplex ureters.
Coronal volume rendered image (A) and Maximum intensity projection image (B) images show horse-shoe kidney with bilateral duplex ureters joining at the midureter level (long arrow) on left side and close to bladder (short arrow) on the right side.
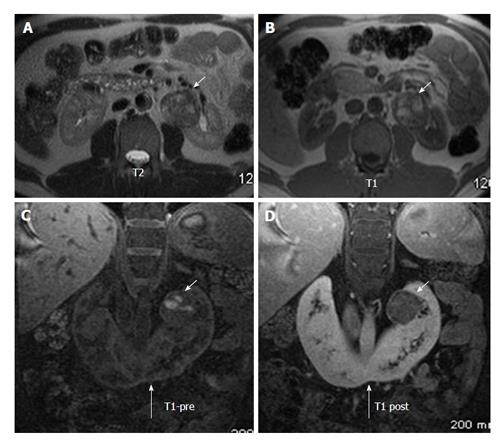
Figure 14 A 43-year-old man on follow up for horse shoe kidney.
Axial T2W (A) and T1W (B) MRI show horse shoe kidney (long arrow) with heterogenous mass in the left renal moiety showing T1 hyperintensity suggestive of blood products. Coronal T1 precontrast (C) and post contrast (D) MRI shows mild enhancement in the renal mass (short arrows). Histopathology came out as renal carcinoid. MRI: Magnetic resonance imaging.
ABNORMAL COLLECTING SYSTEM
Duplex collecting system
A duplex collecting system is one of the most common congenital renal tract abnormalities, characterised by an incomplete fusion of upper and lower pole moieties resulting in a variety of complete or incomplete duplications of the collecting system. Duplication occurs when two separate ureteric buds arise from a single Wolffian duct[31]. Based on the degree of fusion, it can present as bifid renal pelvis, partial ureteric duplication (Y-shaped ureter), incomplete ureteric duplication with ureters joining near or in bladder wall (V-shaped ureter) and complete ureteric duplication with separate ureteric orifices[32,33]. This is often asymptomatic and incidentally detected. USG has limited role in detecting duplication when no hydronephrosis. CTU and MRU are the modality of choice and help in demonstrating the detailed anatomy of the collecting system, level of fusion and status of ureteric orifices[29,34]. Double ureters follow Weigert-Meyer rule (Upper moiety ureter inserts ectopically inferior and medial to the lower pole moiety ureter). Upper moiety often ends in an ureterocele with obstruction and lower pole moiety shows reflux. Because of this upper pole moiety shows hydronephrosis causing mass effect on the lower pole which is seen as drooping lily sign on intravenous urogram[35-37]. Drooping lily sign is a classic urographic sign which refers to the inferolateral displacement of the opacified lower pole moiety due to an obstructed (and unopacified) upper pole moiety[36]. Upper pole moiety ureter can insert into the prostatic urethra in males or vaginal vault in females (Figures 15 and 16).
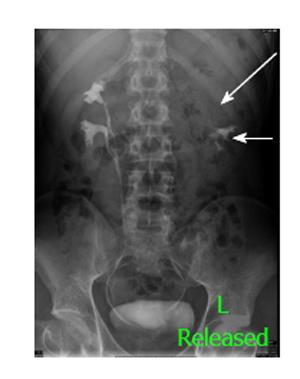
Figure 15 A 25-year-old man with bilateral duplex kidneys and “drooping lily” sign on the left side.
One hour delayed radiograph after computed tomography urogram shows bilateral duplex system with hydronephrotic left upper moiety (long arrow) displacing the lower moiety (short arrow).
Figure 16 Different forms of duplex kidneys based on the level of fusion.
A: Bifid renal pelvis; B: Y shaped ureter; C: V shaped ureter (short arrow). Also seen left lower ureteric stricture (long arrow).
PUJO
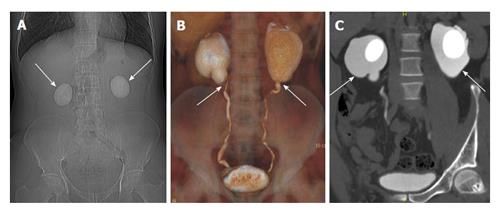
Congenital PUJO is one of the commonest causes of antenatal hydronephrosis reported in 1 per 500 live births[38]. Etiology is debatable and include inadequate canalization during 10-12 wk of gestation, extrinsic obstructions secondary to bands, kinks, and aberrant vessels[39]. Patients can be asymptomatic or present with recurrent urinary tract infections, stone formation or palpable flank mass. Classically intermittent pain after drinking large volumes of fluid or fluids with a diuretic effect is described, due to the reduced outflow from the renal pelvis into the ureter (Dietl’s crisis). Antenatal and postnatal USG are diagnostic and show disproportionately dilated renal pelvis with mild dilatation of calices and non dilated ureters[40]. DTPA is performed to assess the degree of obstruction and split renal function. CT is helpful in demonstrating the crossing vessels before surgical planning[41] (Figure 17). Depending on the degree of obstruction and residual function, surgery is often performed in the form of pyeloplasty or stenting.
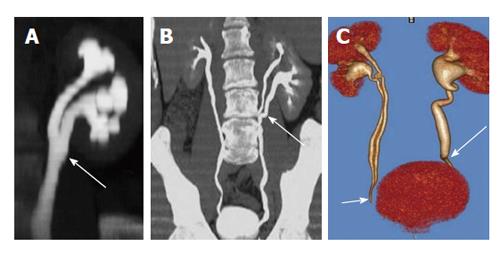
Figure 17 A 24-year-old man with bilateral flank pain.
A: Computed tomography scannogram shows bilateral large round renal calculi; B: Coronal Volume rendered image shows narrowing at bilateral Pelvi-ureteric junction; C: Coronal maximum intensity projection image shows bilateral ballooning of pelvis (arrows) with large round renal pelvic calculi.
CONCLUSION
Congenital anomalies of the kidneys and upper urinary tract encompass a wide spectrum of conditions varying from asymptomatic ectopic kidneys to life threatening renal agenesis (bilateral). US is typically the first imaging performed as it is easily available, non-invasive and radiation free used both antenatally and postnatally. CT and MRI are useful to confirm the ultrasound detected abnormality, detection of complex malformations, demonstration of collecting system and vascular anatomy and more importantly for early detection of complications like renal calculi, infection and malignancies. It is essential for the radiologists to be familiar with the imaging features of wide spectrum of renal anomalies and their complications which will help in timely diagnosis and appropriate management.
P- Reviewer: Cerwenka HR, Lassandro F S- Editor: Song XX L- Editor: A E- Editor: Liu SQ