INTRODUCTION
Head neck cancers (HNC) are the sixth most common cancer worldwide. It is estimated that there are an annual total of 53640 new head neck (oral cavity, pharynx and larynx) cancer cases and 11520 deaths in the United States in 2013[1]. There are many challenges in diagnosis, pretreatment staging and post treatment evaluation in these patients. The clinical signs and symptoms may be nonspecific and can vary depending on the tumor site in the head and neck (oral cavities, pharynx, larynx, nasal cavity, paranasal sinuses, salivary glands, thyroid and skin). Some cancers are so occult that escape detection by detailed physical examination, endoscopy and conventional cross sectional imaging. Contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography/CT (PET/CT) are widely used to determine the presence and extent of the tumors both before and after treatment. However, PET/CT is superior to both CT and MRI in detection of carcinoma of unknown primary (CUP), cervical lymph node metastasis, distant metastasis, residual tumor, recurrent disease and second primary tumors resulting alteration in treatment planning[2-5]. This article aims to review current roles of PET/CT in head neck cancer in both pre treatment staging and post treatment assessment.
PET/CT is useful and approved by the Centers for Medicare and Medicaid Services in patients with HNC for both pre treatment and post treatment phases. Before treatment, PET/CT can be used for delineation of extent of primary tumor (T), detection of an unknown primary tumor origin (T) or synchronous second primary tumor (T), detection of regional lymph node metastasis (N) and detection of distant metastasis (M). After therapeutic treatment, it is proven to be helpful in the assessment of therapy response, detection of residual primary tumor, long-term surveillance for recurrence in both the primary site and metastatic lymph nodes as well as the detection of distant metastases.
PRE-TREATMENT EVALUATION
Standard staging of head and neck squamous cell carcinoma (HNSCC) follows the systematic TNM classification per American Joint Committee on Cancer seventh edition[6]. The primary tumor extension (T stage) varies from site to site given differences in specific anatomic detail of each site, while regional lymph node involvement (N0 to N3 stage) shares similar classification with the exception of thyroid and nasopharyngeal cancers. Metastasis outside head neck regions (e.g., mediastinal and axillary lymph nodes) represents distant metastasis (M stage)[6]. Precise tumor staging is critical for treatment planning and prognosis. Prior to initiation of treatment, HNSCC is clinically staged by using clinical examination, imaging, and endoscopy with tissue biopsy or fine needle aspiration. Multiple studies suggest that PET/CT is superior to conventional imaging (CT or MRI) in initial staging and may alter management and treatment especially when unexpected cervical lymph node and/or distant metastasis is discovered[2,5]. National Comprehensive Center Network issued an update in clinical practice guidelines in head neck cancer and PET/CT imaging in 2013 and suggested using PET/CT for initial staging of the oral cavity, oropharyngeal, hypopharyngeal, glottic, and supraglottic cancers for stage III-IV disease as well as mucosal melanoma and nasopharyngeal carcinoma (World Health Organization class 2-3 and N2-3 diseases)[7].
Primary tumor staging (T)
The T stage of each site is determined by the size of the primary tumor and invasion into the deep structures[6]. Contrast enhanced CT and MRI have been the primary imaging modalities for evaluating T stage of HNSCC due to their superior anatomic resolution and tissue contrast as compare to PET/CT performed without intravenous (IV) contrast. PET/CT performed with IV contrast provides both anatomic and metabolic details at the same time. However, there is no clear recommendation for routine use of PET/CT in initial T staging. Ha et al[2] found that PET/CT upstaged T staging in 2 of 36 patients (5.5%) with subsequent changes in treatment planning. A prospective study by Scott and colleagues showed that PET changed T staging in 6 of 71 patients (8.5%)[5]. MRI remains the preferred imaging method in the assessment of nasopharynx, oral cavity, perineural spread and bone marrow invasion[8,9] while CT is the modality of choice for the larynx and bony cortex invasion[10].
Oral cavity and oropharynx
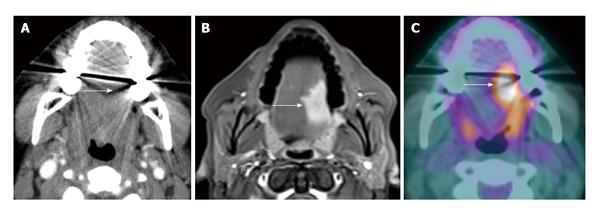
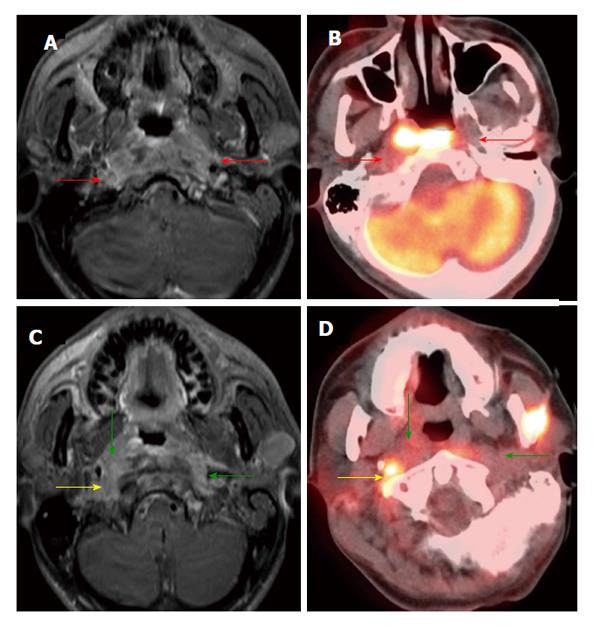
Dental amalgam artifact is a unique problem for CT imaging in the oral cavity and oropharynx. When severe, it can obscure the entire tumor particularly in the oral cavity. PET and MRI are less affected by this type of artifact (Figure 1). MRI, however, is prone to motion artifact given it requires more imaging time. PET/CT also has a high false positivity due to normal lymphoid tissue uptake in the Waldeyer’s ring. Seitz and colleagues did not find additional value of PET/CT to MRI in T staging of oral cavity and oropharyngeal cancers using histopathology as the gold standard. This is thought to be due to limited resolution of PET/CT in detection of small, superficial lesions and lesions obscured by dental artifact[11]. PET/CT has unique benefits in that it can reveal the full tumor extent when the tumor is ill-defined with submucosal extension and diffuse infiltration. This can help the clinician in differentiating tumor border versus normal tissue planes (Figure 2).
Figure 1 Dental amalgam artifact obscuring an oral cavity tumor.
A: The T2 left lateral oral tongue squamous cell carcinoma (arrow) was obscured by the dental amalgam artifact on contrast-enhanced computed tomography (CT); B: The lesion is better evaluated on the contrast-enhanced T1W; C: Positron emission tomography/CT (PET/CT) images. Information from the PET portion is clearly less affected by streak artifact. However, PET/CT does not add additional information to magnetic resonance imaging in terms of primary oral cavity lesion extent for the majority of cases.
Figure 2 Positron emission tomography/computed tomography for delineation of oral cavity tumor extent.
A T4a oral and base of tongue squamous cell carcinoma (red arrows) diffusely and symmetrically infiltrates the ventral aspect of the tongue, whose margins are difficult to appreciate on contrast enhanced computed tomography (CT) (A and B). On the other hand, positron emission tomography/CT (PET/CT) (C and D) clearly demarcates the tumor extent.
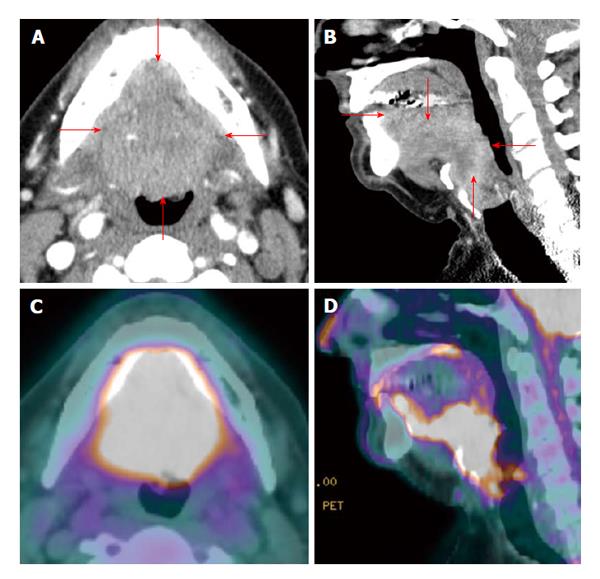
A particular concern in the oral cavity is assessing mandibular/bone involvement. Superficial mandibular involvement by a resectable oral cavity cancer is often treated by marginal mandibulectomy while gross mandibular invasion typically requires segmental mandibulectomy with composite free flap reconstruction[12] (Figure 3). Metabolic activity seen in the mandible tends to overestimate the presence of the tumor due to partial volume averaging and misregistration artifact (Figure 4). The CT portion of the PET/CT and ceCT has more sensitivity and specificity than PET alone in assessment of mandibular invasion[13]. MRI is useful in detection of bone marrow changes as compared to CT. CT is generally superior to MRI in the detection of cortical bone erosion. Overall MRI has similar accuracy as compared to CT and PET/CT. Accuracy is increased when the information of multiple imaging modalities are analyzed if available[14].
Figure 3 Positron emission tomography/computed tomography for detection of mandibular invasion.
A T4a floor of mouth squamous cell carcinoma (white arrow) with through-and-through involvement (red arrow) of the anterior mandible is demonstrated on axial contrast-enhanced computed tomography (CT) (A: Soft tissue window; B: Bone window), axial contrast-enhanced magnetic resonance imaging (C: T1WI; D: Post contrast enhanced T1WI with fat saturation), and positron emission tomography/CT (E: Axial; F: Sagittal).
Figure 4 Shine-through artifact on positron emission tomography/computed tomography.
A T4a floor of mouth squamous cell carcinoma (arrow) appeared to extend to involve the lingual cortex of the mandible seen on positron emission tomography/computed tomography (CT) (A), which is in fact artifactual. The corresponding CT (B) bone window and intraoperative findings confirmed an intact mandibular cortex.
Routinely CT is performed with the patient’s mouth close and in neutral position. Apposition of oral cavity and oropharyngeal structures can obscure the tumors. CeCT with dynamic maneuvers (puffed cheek technique, open mouth position or modified Valsava maneuver) can delineate the presence and extent of the lesions[15]. The puffed cheek technique performed during PET/CT scanning has been proven to be practical and improved lesion detection[16].
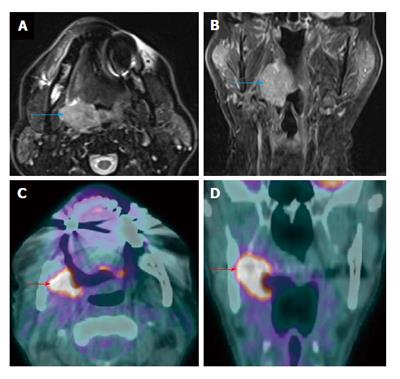
Nasopharynx
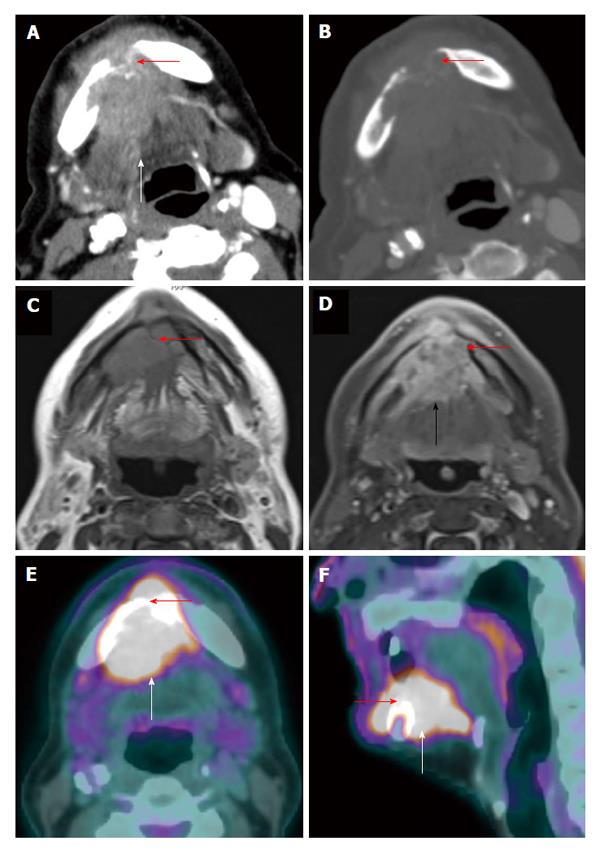
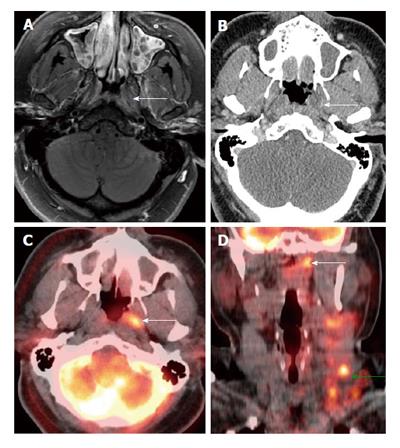
MRI is known to be superior to PET/CT for the assessment of locoregional invasion (parapharyngeal space, skull base, intracranium, sphenoid sinus) and retropharyngeal nodal metastasis by nasopharyngeal carcinoma[9]. PET/CT tends to underestimate the extent and volume of tumor in the nasopharynx, skull base, brain and orbits as compare to MRI (Figure 5) which could have significant impact on staging or treatment planning especially when the discordance is outside the nasopharynx[17]. Similar limitations of PET/CT are also noted in pediatric nasopharyngeal carcinoma patients[18]. However PET/CT can be very helpful in identification of a subtle but focally hypermetabolic nasopharyngeal carcinoma when CT and MRI findings are not obvious (Figure 6). In addition PET/CT has the advantage of screening for distant metastatic disease.
Figure 5 Positron emission tomography/computed tomography underestimates size and extension of the nasopharyngeal carcinoma and retropharyngeal lymph nodes compared to magnetic resonance imaging.
The approximate lateral extension (red arrows) of the nasopharyngeal carcinoma is well demarcated on magnetic resonance imaging (MRI) (A). The tumor appears to be smaller on positron emission tomography/computed tomography (CT) (B) as compared to MRI because the lateral portion of the tumor is hypometabolic. Inferior to the primary tumor site, there is metastatic bilateral retropharyngeal lymphadenopathy (yellow and green arrows). The full extent of retropharyngeal lymph node involvement is better assessed by MRI (C) than CT (D). Only the right lateral retropharyngeal node is FDG avid.
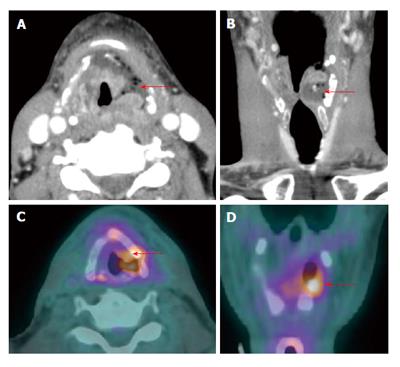
Figure 6 Positron emission tomography/computed tomography for detection of small submucosal nasopharyngeal carcinoma when computed tomography and magnetic resonance imaging are unrevealing in a patient presenting with metastatic cervical lymphadenopathy.
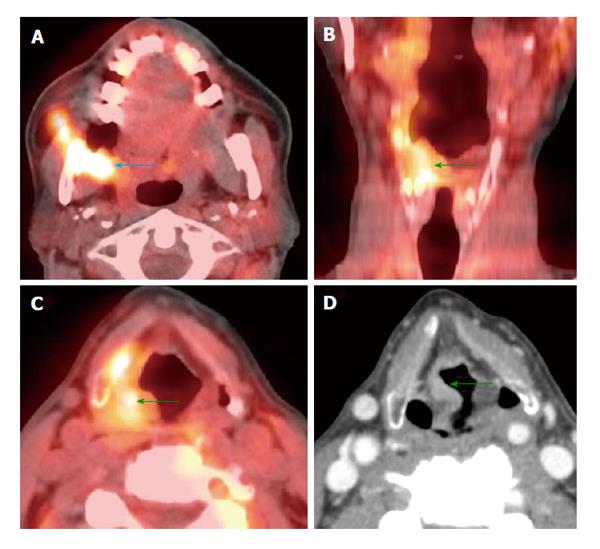
Endoscopy was unremarkable. There was slight enhancement in the left side of the nasopharynx (white arrows) seen on magnetic resonance imaging (A) without abnormality on computed tomography (CT) (B). Positron emission tomography/CT (PET/CT) (C and D) revealed a focal hypermetabolic region in the left side of the nasopharynx (white arrows), suspicious for a submucosal tumor. Hypermetabolic left-sided cervical lymphadenopathy was also noted (green arrow). Biopsy revealed squamous cell carcinoma at the site of hypermetabolic activity in the left nasopharynx directed by PET/CT.
Larynx
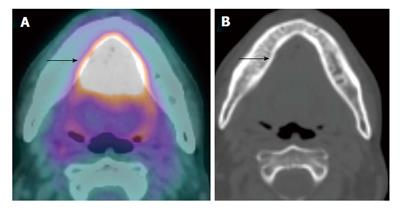
In primary laryngeal tumors the invasion of the preepiglottic fat and the paraglottic fat by laryngeal carcinoma (T3) increases the risk of regional lymph node metastasis. These tumors tend to have a higher recurrence rate and worse outcome when they invades the thyroid or cricoid cartilages, particularly when full thickness cartilage invasion (T4a) occurs[19]. CT is more specific while MRI is more sensitive in detection of cartilage invasion[10]. PET/CT without contrast adds no additional information to CT or MRI with IV contrast (Figure 7). However, pretreatment PET/CT with IV contrast is valuable as a baseline study to compare to the post treatment study[20].
Figure 7 Laryngeal cartilage invasion.
A large T4a squamous cell carcinoma of the larynx with through-and-through cricoid cartilage invasion (blue arrow) and extralaryngeal invasion (red arrow) demonstrated on both positron emission tomography/computed tomography (CT) (A) and contrast-enhanced CT (B).
Perineural spreading
Perineural spreading occurs when the tumor spreads along the peripheral nerve away from the primary tumor site, and is associated with poor outcomes. The patient may be asymptomatic and it may not be detected during surgery[21]. MRI is the study of choice in detection of perineural spread due to its high tissue contrast. It is important for radiologists to be familiar with PET/CT findings of perineural spreading as some patients may not have MRI as the initial or post treatment evaluation. On PET/CT, perineural spread can present with abnormal linear or curvilinear hypermetabolic activity along the trigeminal or facial nerves[8] (Figure 8).
Figure 8 Perineural spread.
Positron emission tomography/computed tomography (A: Axial; B: Coronal; C: Axial; D: Sagittal) demonstrates a T4b right oropharyngeal squamous cell carcinoma (green arrow) spreading along the mandibular branch of the trigeminal nerve (yellow arrow) through the right foramen ovale (red arrows) to involve the right cavernous sinus (black arrows). The left mandibular nerve in the foramen ovale is normal (blue arrow).
Detection of CUP origin
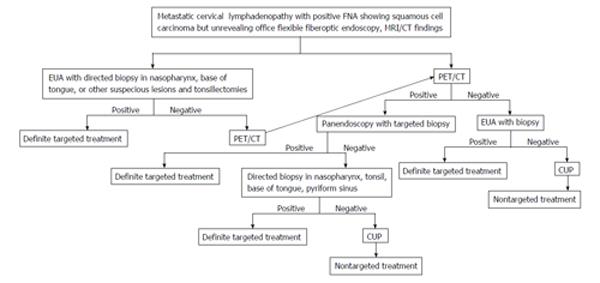
Two to 7% of HNSCC patients present with metastatic cervical lymphadenopathy without definite primary sites[22,23] detected by a complete history (nonspecific symptoms or no symptoms), thorough physical examination/office flexible fiberoptic endoscopy (small submucosal lesion), or conventional contrast enhanced CT/MRI (small lesion obscured by normal lymphoid tissue). The work up algorithm to search for the primary tumor is shown in Figure 9.
Figure 9 Algorithm in diagnosis and management of carcinoma of unknown primary.
MRI: Magnetic resonance imaging; PET/CT: Positron emission tomography/computed tomography; CUP: Carcinoma of unknown primary.
The choice of treatment depends on staging and histology[24]. Failure to identify the primary tumor leads to nontargeted treatment (bilateral tonsillectomies, bilateral neck dissection, radiation to cover the whole pharyngeal mucosa and neck)[25] resulting in increased complications, morbidity and mortality.
Various studies show that PET/CT is able to identify the primary cancer 29% to 54% of cases with 62% to 93% sensitivity, 33% to 93% specificity, 56% to 89% positive predictive value (PPV) and 25% to 96% negative predictive value (NPV)[3,26-31]. The majority of the primary cancers are found in the palatine tonsils or base of tongue[32] (Figure 10). A high detection rate of up to 54% can be achieved when the combination of CT, MRI, endoscopy under anesthesia and PET/CT are used. Due to variable negative predictive value of PET/CT, otolaryngologists should still strongly consider panendoscopy with directed biopsy and bilateral tonsillectomies when PET/CT yields negative result[3]. Radiologists should be aware of recent panendoscopy with biopsy before PET/CT. Recent biopsy can cause false-positive result as high as 50%[30]. It is still uncertain when PET/CT should be performed after biopsy to avoid false positivity, therefore if carcinoma of unknown primary is suspected it is best to obtain PET/CT prior to endoscopy and biopsy/tonsillectomy[26,33]. Obtaining PET/CT prior to biopsy may alert the clinician to suspected primary tumor sites and avoid the false positive imaging result if PET/CT is obtained after biopsy.
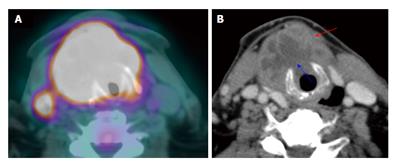
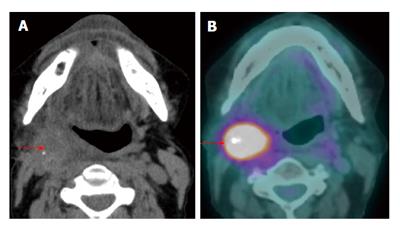
Figure 10 Carcinoma of unknown primary.
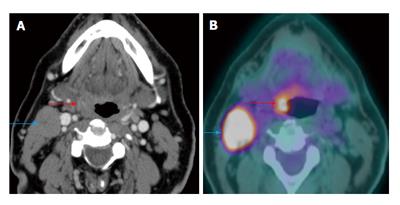
The patient presented with palpable right level IIa lymphadenopathy (blue arrows). Fine needle aspiration of the lymph node was positive for squamous cell carcinoma. Panendoscopy without biopsy and contrast-enhanced computed tomography (CT) (A) were unremarkable. Positron emission tomography/CT (B) showed a small hypermetabolic area in the right palatine tonsil (red arrows) proven to be squamous cell carcinoma by biopsy.
Identification of second primary malignancy
Synchronous second primary malignancy (SPM) occurs within 6 mo when the index primary cancer is detected. Approximately 1.4% to 18%[34] of head neck cancer patients have SPM, especially when the index cancers are laryngeal carcinomas. Most synchronous SPM are found in the lung (Figure 11), head and neck region itself (Figure 12) or esophagus[35] with the majority of the tumors being squamous cell carcinoma[36]. A recent study found that human papillomavirus (HPV)-seropositive patients, especially those who never smoked, have less risk of developing SPM as compared to HPV-seronegative group[37]. Since SPM is one of the main causes of death in early-stage HNSCC, early detection and treatment of SPM increases survival[34]. Detection of SPM also changes treatment planning[33]. A meta-analysis revealed 87.5% sensitivity and 95% specificity of PET/CT in detection of SPM or distant metastasis[38]. Negative PET/CT does not completely exclude the presence of SPM[38].
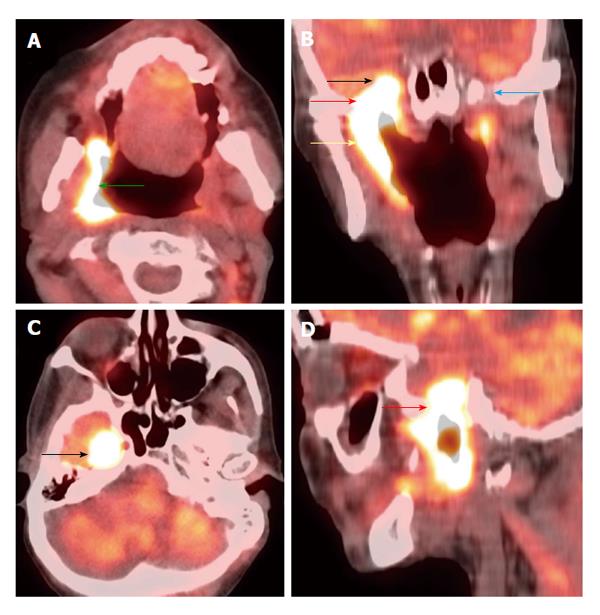
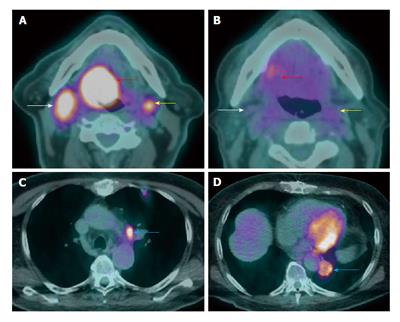
Figure 11 Synchronous second primary malignancy in the lung.
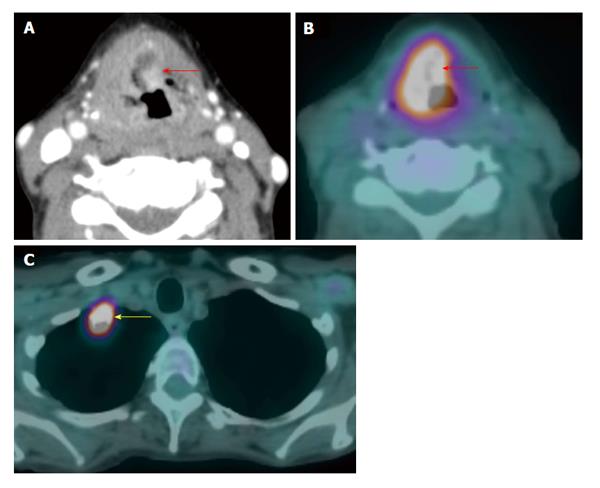
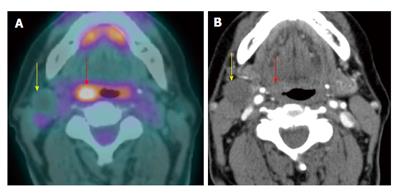
A T4a supraglottic squamous cell carcinoma (red arrows) with extralaryngeal invasion is well demonstrated on computed tomography (CT) (A) and positron emission tomography/CT (B). There is a 3-cm hypermetabolic second primary squamous cell carcinoma (yellow arrow) in the right lung apex, discovered at the same time in this patient who had an extensive smoking and drinking history. The rest of the exam is unremarkable.
Figure 12 Synchronous second primary malignancy in the head and neck region.
A T4b right retromolar trigone squamous cell carcinoma involving the masticator space (blue arrow) was seen on positron emission tomography/computed tomography (PET/CT) (A). There was another second primary tumor in the right aryepiglottic fold (green arrows) causing no symptoms, demonstrated by both PET/CT (B and C) and contrast-enhanced CT (D).
Lymph node involvement (N)
The likelihood of cervical lymph node metastasis in HNSCC depends on location, histology and staging of the primary tumor[39]. The presence of metastatic nodes carries poor prognosis, decreases 5-year survival and necessitates treatment in the neck[40,41]. Differentiation between metastatic lymph nodes and reactive nodes by CT/MR size and morphologic criteria can be difficult[42]. A meta-analysis by Kyzas et al[39] showed higher sensitivity/specificity (80%/86%) of PET/CT as compared to other conventional diagnostic modalities (75%/79%). The higher sensitivity is due to the fact that PET can show hypermetabolism in normal-sized metastatic lymph nodes. However PET is not 100% specific because inflammatory reactive nodes and adjacent granulation tissue can increase uptake[43] yielding a false positive result. Radiologists should be aware that small necrotic lymph nodes may appear hypometabolic on PET especially in hyperglycemic patients[44]. Therefore it is important to analyze both morphologic and metabolic information derived from PET/CT (Figure 13).
Figure 13 Hypometabolic necrotic lymph node.
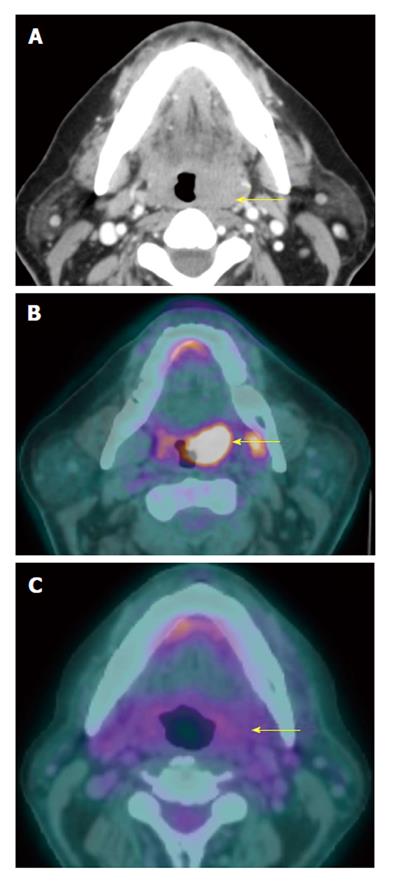
A T1N1M0 right glossopharyngeal sulcus squamous cell carcinoma (red arrow) is FDG avid on positron emission tomography/computed tomography (PET/CT) (A). There is a 1.7-cm necrotic right level IIa lymph node seen on contrast-enhanced CT (B). The necrotic lymph node is hypometabolic and may potentially cause a false negative result if the CT portion of the PET/CT or corresponding contrast-enhanced CT were not correlated.
HNSCC patients whose metastatic lymph nodes are not palpable on physical examination or visualized on imaging are determined to be clinically N0 (cN0) stage[6]. PET/CT failed to detect half of the cN0 which were confirmed to have metastasis by histopathology. This is thought to be due to small size of occult metastasis beyond resolution of PET (typically micrometastases < 5 mm), obscuration by physiologic glucose uptake in adjacent organs or hyperglycemia. Therefore it is not recommended to routinely use PET/CT to assess possible cervical lymph node metastasis in cN0 setting[39]. However, pretreatment PET/CT may still be useful as an optional imaging to compare with the post treatment scan to discriminate malignant tissue from physiologic uptake[45].
Distant metastasis (M)
Approximately 7% to 25% of patients with advanced stage HNSCC have distant metastases at initial presentation[46]. The most common sites of metastasis include lung (Figure 14), bone and abdomen. Mediastinal lymph node involvement is considered distant metastasis[6]. Contrast enhanced chest CT is commonly used to assess intrathoracic spread with 73% sensitivity and 80% specificity[47]. Overall PET/CT is more accurate than conventional imaging in detecting metastatic foci[48]. A meta-analysis by Xu et al[49] revealed sensitivity and specificity of PET/CT around 87.5 % and 95% respectively. It is very important to detect distant metastases early in the workup as it changes prognosis and management. Extensive surgery with curative intent may cause significant morbidity and mortality and may be avoided in the event of documented distant metastases. PET/CT is recommended when distant spread is suspected in HNSCC patients with locoregionally advanced stage. However, negative PET/CT does not completely exclude absence of metastasis[49].
Figure 14 Lung metastasis at initial staging.
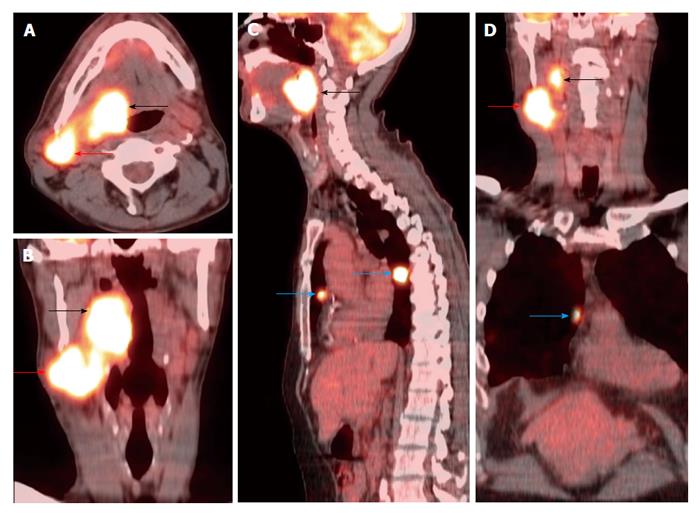
A T3N2bM1 right base of tongue squamous cell carcinoma (black arrows) was FDG avid on positron emission tomography/computed tomography (A-D) with metastasis to the right level IIa (red arrows) and lung (blue arrows).
Change in initial staging and management
Lonneux et al[50] performed a multicenter prospective study to evaluate the impact of PET/CT on the initial staging and management of patients with HNSCC. The group found that PET/CT improved the TNM classification of the disease and altered the management of 13.7% of the patients mainly due to the ability of PET/CT to detect metastatic or additional disease.
POST TREATMENT EVALUATION
Therapy response assessment and residual tumor detection
A small early-stage HNSCC (e.g., a T1N0 true vocal cord carcinoma) can be treated with a single modality[51]. Advanced-staged disease (Stage III and IV) typically needs multimodality treatments including surgery, radiation and chemotherapy[52]. Radical concurrent chemoradiation can be used as a definite therapy in preference to surgery to achieve similar cure rates with less morbidity and to preserve organ function[53]. Surgery and radiation can cause significant inflammation, fibrosis and distortion of the anatomy preventing accurate differentiation between residual tumor and complete tumor response by conventional imaging[54,55]. Inaccurate post treatment assessment may cause delayed or unnecessary treatment resulting in increased mortality and morbidity[45]. Multiple studies have shown that PET/CT is superior to conventional anatomic imaging in assessment of tumor response and detection of residual tumor. This is helpful to the surgeon in selecting the appropriate patients for salvage surgery after chemoradiation[4,56-58].
The sensitivity, specificity, PPV and NPV of PET/CT for detection of residual primary tumor were 94%, 82%, 75% and 95% respectively[4]. It is noted that PET/CT has very high NPV, therefore negative result highly suggests absence of viable residual disease in both primary site and neck (Figures 15 and 16). The low PPV is due to treatment-related FDG-avid inflammation or infection (Figure 17). A positive PET/CT result in the post treatment phase needs careful correlation with clinical information and corresponding CT/MRI findings[45]. It is suggested that PET/CT should be performed no sooner than 2 mo after completion of treatment to avoid false positive results; however it may be performed sooner if there is clinically suspected recurrent disease[59]. We generally recommend performing PET/CT around 3 mo after completion of treatment at our institution.
Figure 15 Complete treatment response at the primary tumor site.
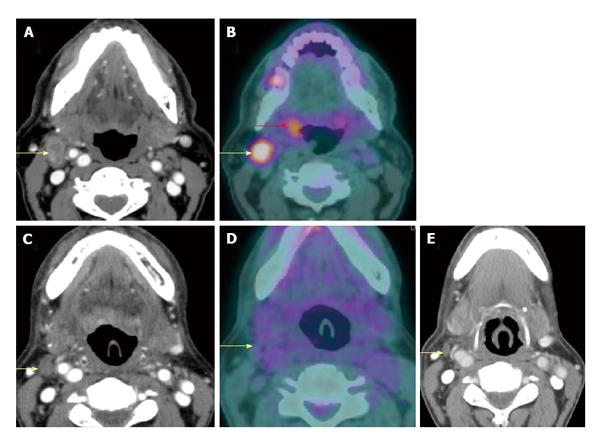
A T2N0M0 oral tongue squamous cell carcinoma (yellow arrow) seen on contrast-enhanced computed tomography (CT) (A) and positron emission tomography/CT (PET/CT) (B) showed complete response after 3 mo of chemoradiation demonstrated on PET/CT (C). The tumor is no longer hypermetabolic. The patients remained disease free for 3 years, proving true negative result of PET/CT.
Figure 16 Complete response of a metastatic lymph node.
A T2N2bM0 right palatine tonsil squamous cell carcinoma (red arrow) and right level IIa metastatic lymph node (yellow arrows) were seen on both contrast-enhanced computed tomography (CT) (A) and positron emission tomography/CT (PET/CT) (B). After chemoradiation, the node was smaller on contrast-enhanced CT at 6 wk (C) and hypometabolic on PET/CT at 4 mo (D), representing complete response to treatment. Negative physical examination and PET/CT at 1 year (E) confirmed true negative result of the PET/CT performed at 4 mo.
Figure 17 False positive positron emission tomography/computed tomography result due to post treatment infection.
A T3N0M0 left supraglottic squamous cell carcinoma showed edema and necrosis at the tumor site (red arrows) on contrast-enhanced computed tomography (CT) (A and B) at 5 wk after chemoradiation and increased uptake on positron emission tomography/CT (PET/CT) (C and D) at 10 wk. The patient had severe throat pain and fever. A residual tumor can not be excluded, therefore endoscopy with biopsy was performed showing radiation-induced inflammation, tumor necrosis and superimposed actinomycosis causing a false positive result on PET/CT. Due to lack of viable tumor, no salvage surgery was performed. The patient was disease free at two-year follow up.
Long-term surveillance
The purpose of obtaining PET/CT as a surveillance tool is to allow for the early detection of recurrent disease (both in the primary site and the neck) (Figure 18), assess for a metachronous second primary tumor (Figure 19) and to rule out distant metastases (Figure 20). PET/CT has 93% to 100% sensitivity and 63% to 94% specificity in detection of recurrent tumor in both primary site and the neck respectively[45,60,61]. The NPV of a single PET/CT and double PET/CT (obtained within 6 mo period) are 91% and 98% respectively. Negative results of two consecutive PET/CT studies could potentially eliminate the need for routine post treatment imaging if there is no clinical suspicion of tumor recurrence[62]. In addition there are no differences in survival between PET/CT detected and clinically detected recurrences[63]. Although there is an appreciable radiation dose and lifetime cancer risk associated with PET/CT, the use of this examination is warranted when utilized in the appropriate clinical setting[64].
Figure 18 Recurrent primary tumor detected by positron emission tomography/computed tomography.
A T3N0M0 right palatine tonsil squamous cell carcinoma was demonstrated on axial T2W magnetic resonance imaging (MRI) (blue arrow) (A) and coronal contrast-enhanced T1W MRI (B). MRI and positron emission tomography/computed tomography (PET/CT) performed 2 and 3 mo after completion of chemoradiation demonstrated complete treatment response (not shown). Surveilance PET/CT (C and D) revealed intense increase metabolism in the right palatine tonsil and medial pterygoid muscle (red arrows) representing a recurrent squamous cell carcinoma.
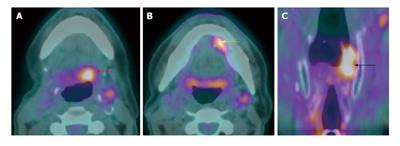
Figure 19 Recurrent nodal disease.
A T2N1M0 right hypopharyngeal squamous cell carcinoma status post completed chemoradiation 2.5 years ago with complete response (not shown). The patient presented with right sided level II lymphadenopathy. Computed tomography (CT) without contrast (A) showed an ill-defined mass in the right level II. Positron emission tomography/CT (PET/CT) (red arrow) (B) demonstrated very intense metabolism in the mass. Biospy confirmed a recurrent squamous cell carcinoma in the right level IIa node.
Figure 20 Failure of treatment due to distant metastasis.
A T4aN2cM0 right base of tongue squamous cell carcinoma (red arrow) with bilateral level IIa metastatic lymphadenopathy (white and yellow arrows) seen on positron emission tomography/computed tomography (PET/CT) at initial staging (A and B). The patient received chemoradiation. PET/CT (C and D) performed at 3 mo after treatment showed new lung metastases (blue arrow).
Metachronous second primary tumor may occur after 6 mo of the index primary tumor with 2.8% annual rate[65] (Figure 21). The incidence of distant metastasis following definitive treatment is 9% with the risk increased in patients with locally advanced stages[65,66]. Overall 17.9% of HNSCC patients develop second primary cancers and/or distant metastasis, especially in patients with recurrent diseases[38,66]. The identification of distant metastatic lesions at the time of restaging recurrent tumors may allow the clinician to avoid aggressive surgery and focus on palliative chemoradiation options[66]. PET/CT has strong utility in detecting second primary tumors or distant metastases with a sensitivity and specificity of 88.8% and 95.1% respectively[38].
Figure 21 Metachronous second primary tumor.
A T1N0M0 left floor of mouth squamous cell carcinoma (SCC) (not shown) s/p wide local excision with primary closure and selective neck dissection 3 yr ago. Surveilance positron emission tomography/computed tomography (A-C) showed a recurent SCC at left floor of mouth (yellow arrow) and two metachronous second primaries at the left hypopharyngx (black arrow) and left base of tongue (red arrow).