Published online Dec 28, 2012. doi: 10.4329/wjr.v4.i12.455
Revised: August 23, 2012
Accepted: August 30, 2012
Published online: December 28, 2012
AIM: To compare the efficacy and safety of bronchial artery embolization (BAE) with n-butyl cyanoacrylate (NBCA) and gelatin sponge particles (GSPs).
METHODS: Six healthy female swine were divided into two groups to be treated with BAE using NBCA-lipiodol (NBCA-Lp) and using GSPs. The occlusive durability, the presence of embolic materials, the response of the vessel wall, and damage to the bronchial wall and pulmonary parenchyma were compared.
RESULTS: No animals experienced any major complication. Two days later, no recanalization of the bronchial artery was observed in the NBCA-Lp group, while partial recanalization was seen in the GSP group. Embolic materials were not found in the pulmonary artery or pulmonary vein. NBCA-Lp was present as a bubble-like space in bronchial branch arteries of 127-1240 μm, and GSPs as reticular amorphous substance of 107-853 μm. These arteries were in the adventitia outside the bronchial cartilage but not in the fine vessels inside the bronchial cartilage. No damage to the bronchial wall and pulmonary parenchyma was found in either group. Red cell thrombus, stripping of endothelial cells, and infiltration of inflammatory cells was observed in vessels embolized with NBCA-Lp or GSP.
CONCLUSION: NBCA embolization is more potent than GSP with regard to bronchial artery occlusion, and both materials were present in bronchial branch arteries ≥ 100 μm diameter.
- Citation: Tanaka T, Kawai N, Sato M, Ikoma A, Nakata K, Sanda H, Minamiguchi H, Nakai M, Sonomura T, Mori I. Safety of bronchial arterial embolization with n-butyl cyanoacrylate in a swine model. World J Radiol 2012; 4(12): 455-461
- URL: https://www.wjgnet.com/1949-8470/full/v4/i12/455.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i12.455
Bronchial artery embolization (BAE) for patients with massive hemoptysis has been an established treatment procedure since Rémy et al[1] reported the efficacy of BAE in 1974. Particles such as gelatin sponge (GS) or polyvinyl alcohol (PVA) have been used as embolic materials[2-5]. Problems with BAE include recurrent hemoptysis, and although very rare, ischemic spinal paraplegia[6,7]. The occurrence of paraplegia is considered to be related to the anatomical anastomosis between the bronchial artery and the anterior spinal artery. Furthermore, Ivanick et al[8] have reported that BAE using ethanol with high occlusion potency causes bronchial wall necrosis.
Currently, BAE with n-butyl cyanoacrylate (NBCA) instead of particles is reported to enhance the potency of the occlusion, aiming to reduce the incidence of recurrent hemoptysis[9,10]. It is anticipated that peripheral occlusion with higher potency BAE causes damage to the bronchial wall and pulmonary parenchyma. Nonetheless, only one case has been found of bronchial wall damage following BAE with NBCA. We thought that it was important to establish the safety mechanism of BAE with NBCA.
The purpose of this study was to compare the efficacy and safety of BAE with NBCA and GS particles (GSPs) in a swine model.
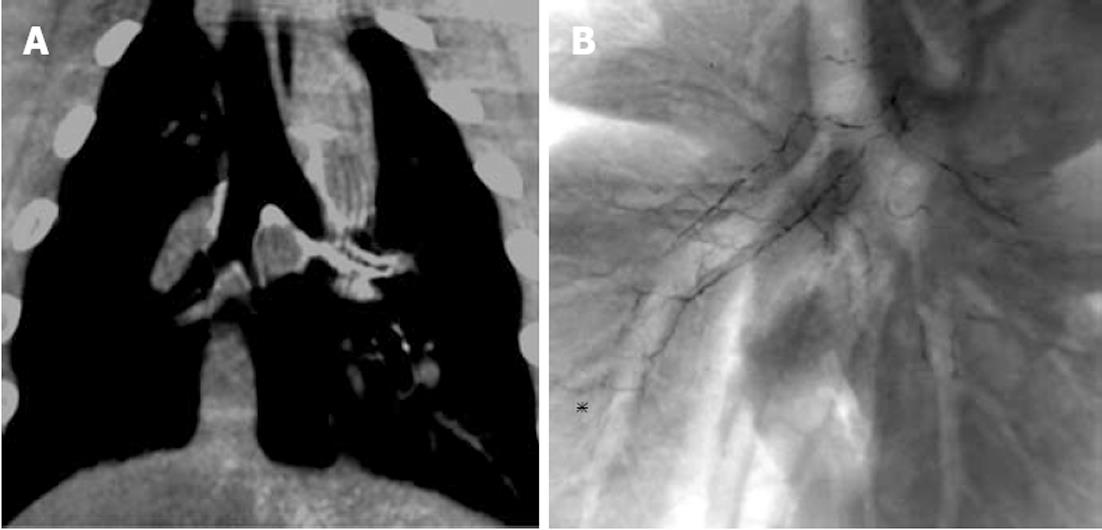
Approval by our Institutional Committee on Research Animal Care was obtained before the study was initiated. We used six healthy female pigs weighing 56-65 kg. The six pigs were divided into two groups with NBCA-Lp or GSPs, with three animals per group. Swine have a common trunk of the right and left bronchial arteries. Embolization of the right and left bronchial arteries was conducted using NBCA-Lp or GSPs, with six embolized lungs per group.
Pre-anesthesia was achieved with a combination of 5 mg/kg ketamine and 0.08 mg/kg atropine sulfate. General anesthesia was maintained with isoflurane gas via intubation. Cardiac and respiratory parameters were monitored throughout the procedures. Each pig underwent embolization of both the right and left bronchial arteries. A 4 Fr sheath (SuperSheath; Medikit, Tokyo, Japan) was inserted by direct puncture into the right femoral artery. Catheterization of the bronchial arterial trunk was performed using a 4 Fr Mikaelsson catheter (Medikit) or a 4 Fr Cobra catheter (Medikit). Selective bronchial trunk angiography was performed via this catheter before and after embolization. A 2.5 F microcatheter (Renegade-18; Boston Scientific, Natick, MA, United States) was coaxially inserted into the right or left bronchial artery and advanced to a peripheral site 1-2-cm from the bifurcation using a 0.014-inch micro-guidewire (Transend EX; Boston Scientific).
BAE using NBCA-Lp or GSPs was then conducted to embolize the bronchial artery. In BAE with NBCA, NBCA was prepared as a liquid embolic material by mixing 1 mL NBCA with 7 mL Lipiodol using a three-way stopcock. The ratio most commonly used for peripheral embolization in previous reports was 1 mL NBCA to 4 or 5 mL lipiodol[11-13]. We speculated that the more diluted NBCA was with Lipiodol, the more easily it should reach the more peripheral sites. Baltacioğlu et al[14] used and 1 to 7 ratio of NBCA-Lp for BAE. Therefore, a ratio of 0.1 mL NBCA to 0.7 mL Lipiodol was adopted in this study. Before embolization, the microcatheter was flushed with 5% glucose solution to prevent polymerization of NBCA. NBCA-Lp was slowly injected through the microcatheter under fluoroscopic control. The excessive NBCA-Lp volume was anticipated to reflux into the aorta. Based on our experiences[15], 0.2 mL NBCA-Lp volume was determined to be injected into the bronchial artery. Actually, 0.2 mL NBCA-Lp was appropriate to arrest blood flow in the bronchial artery without reflux. The microcatheter was removed after each BAE with NBCA-Lp because the lumen occluded instantly. The new microcatheter was coaxially inserted via a 4 Fr catheter placed in the common trunk and introduced to another bronchial artery and advanced a few centimeters peripherally beyond the origin of the bronchial artery, followed by BAE with NBCA.
In BAE with GSPs, GSP (Spongel, Astellas Pharmaceutical Inc., Tokyo, Japan) was cut into 1-mm pieces and soaked in contrast medium (Iopamidol 370; Bracco, Milan, Italy). We previously confirmed that magnified glass view of 53 GSPs revealed that GCPs was composed of four-angled-form sized 0.8-1.6 mm[16]. GSPs were slowly injected through the microcatheter under fluoroscopic control until bronchial arterial flow was arrested. The microcatheter was then introduced into another bronchial artery followed by BAE with GSP. In each group, angiography before and immediately after embolization was conducted to confirm the arrest of bronchial arterial blood flow.
Adverse effects on the lung were evaluated and blood tests were conducted. Peripheral blood was taken before embolization and 1 and 2 d after embolization to assess changes in white blood cells, red blood cells, SpO2 and rectal temperature.. Cone beam computed tomography (CT) (Allraura, Xperfd 20; Philips, Netherlands) was performed to evaluate pulmonary infarction, damage to the bronchial wall in both groups, and the presence of NBCA-Lp, before, immediately after, and 24 h and 48 h after embolization.
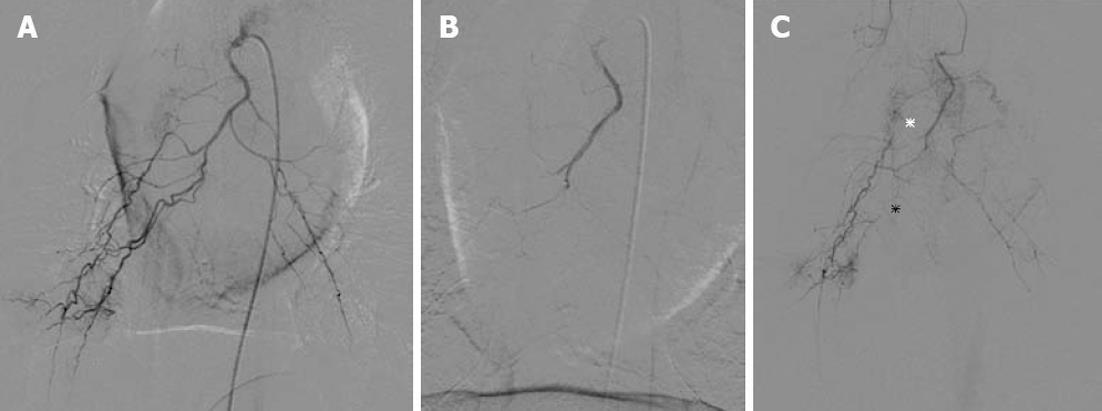
Bronchial arteriography 2 d after BAE was attempted to evaluate the recanalization of the bronchial artery, and compared with the angiographic findings before BAE. When catheterization from the femoral artery approach was difficult, the carotid approach was tried. Damage of the bronchial artery was assessed according to the criteria of Maeda et al[17] into three grades: Grade I, no damage or mild vessel wall irregularity; Grade II, overt stenosis; and Grade III, occlusion. Grade II and III were considered to indicate significant bronchial artery disorder.
The swine were sacrificed with intravenous injection of pentobarbital sodium 2 d after BAE and investigated for evidence of histological pulmonary infarction and/or bronchial mural necrosis. Necropsies were performed and the lungs were removed. The lungs were cut into sagittal sections of 10 mm thickness to follow the bronchial trees, and fixed in a 7.5% neutral formaldehyde buffer. Specimens of 2 cm × 3 cm for microscopic examination were removed from the main/lobar bronchus, segmental bronchus, subsegmental bronchus, or peripheral bronchus branch arteries. The surface of the slice of interest was stained with hematoxylin-eosin (HE) to investigate damage to the bronchus and the pulmonary parenchyma, to identify embolic material in the artery, and to evaluate the vital response to the embolus. Macroscopic and microscopic studies regarding the cutting and the ischemic damage of bronchus and lung were conducted under the direction of a pathologist.
All pigs were able to run in the period after awakening from anesthesia until sacrifice, which implied that BAE with GSPs or NBCA did not cause ischemic damage to the spinal cord. Follow-up data revealed that all the values for white blood cells, red blood cells, SpO2 and rectal temperature did not differ from baseline (Table 1).
| NBCA | GSP | |||||||
| Baseline | Immediately after | 1 d after | 2 d after | Baseline | Immediately after | 1 d after | 2 d after | |
| WBC (× 103/μL) | 16.5 ± 2.8 | 14.1 ± 0.45 | 15.5 ± 2.1 | 17.5 ± 5.3 | 16.3 ± 1.1 | 14.9 ± 0.35 | 17.0 ± 1.75 | 16.0 ± 1.6 |
| RBC (× 106/μL) | 6.04 ± 0.82 | 5.98 ± 0.87 | 6.18 ± 0.10 | 5.69 ± 0.56 | 6.28 ± 0.46 | 6.27 ± 0.52 | 6.21 ± 0.19 | 6.30 ± 0.06 |
| SpO2 (%) | 97.3 ± 1.25 | 97.7 ± 0.94 | 96.7 ± 0.94 | 97.0 ± 0.82 | 96.7 ± 0.47 | 97.3 ± 1.25 | 98.0 ± 0.00 | 97.7 ± 0.47 |
| Rectal temperature (°C) | 37.7 ± 0.45 | 37.8 ± 0.62 | 37.2 ± 0.12 | 37.8 ± 0.60 | 38.2 ± 0.41 | 38.4 ± 0.45 | 37.8 ± 0.65 | 37.9 ± 0.63 |
In the NBCA-Lp group, radiography and CT immediately and 2 d after embolization revealed accumulation of NBCA-Lp in the principal, lobar, segmental and subsegmental branch arteries (Figure 1, Table 2). In both groups, radiography and CT revealed no specific changes in the pulmonary area.
| Bronchial arteries | NBCA | |
| Present | Absent | |
| Principal branch | 6 | 0 |
| Lobar branch | 13 | 5 |
| Segmental branch | 12 | 47 |
| Subsegmental branch | 3 | 123 |
In the NBCA-Lp group, selective angiography 2 d after BAE could not be performed because it was impossible to catheterize each bronchial artery using the femoral or common carotid approach due to severe stenosis or occlusion. In the GSP group, the follow-up bronchial catheterization and angiography were possible and revealed partial recanalization of the bronchial artery with overt stenosis and/or occlusion of the bronchial branch arteries (Figure 2, Table 3).
Macroscopic examination revealed congestion and edema throughout the resected lungs but no coagulation necrosis in the bronchial wall and pulmonary parenchyma. Microscopy also revealed no specific changes in the pulmonary area and bronchial tree wall in either group.
The presence of NBCA-Lp and GSPs was investigated microscopically in 24 and 53 specimens, respectively. It was difficult to detect GSPs; probably because they were dispersed in the vessel and the number of the GSP specimens was greater than the NBCA-Lp specimens.
NBCA-Lp and GSPs were found in the bronchial branch arteries but not in the pulmonary artery or pulmonary vein. Principal (720-1240 μm), lobar (407-700 μm), segmental (142-413 μm) and subsegmental (40-184 μm) branch arteries were observed. These arteries were in the adventitia outside the bronchial cartilage. Meanwhile, numerous fine vessels < 50 μm in diameter were observed in the submucosal and cartilage layers (Figure 3). NBCA-Lp or GSPs were found in the principal bronchus branch arteries to subsegmental branch arteries. NBCA-Lp and GSPs were present in 35 and six vessels, respectively. The diameters of NBCA-LP present in bronchial branch arteries were 127-1240 μm (Table 4), and that of GSPs were 107-853 μm. Either NBCA-Lp or GSPs were identified in bronchial branch arteries ≥ 100 μm in the adventitia. No NBCA-Lp or GSPs were found in vessels < 100 μm, including numerous fine vessels inside the bronchial cartilage (Figure 3A).
| Diameter of bronchial branch artery (μm) | NBCA-Lp | GSP | ||
| Present | Absent | Present | Absent | |
| 1000 < | 1 | 1 | ||
| 951-1000 | ||||
| 901-950 | 2 | |||
| 851-900 | 1 | |||
| 801-850 | 1 | 1 | ||
| 751-800 | ||||
| 701-750 | 2 | |||
| 651-700 | 2 | 1 | ||
| 601-650 | 1 | |||
| 551-600 | 1 | 1 | 3 | |
| 501-550 | 3 | 1 | ||
| 451-500 | 1 | 1 | ||
| 401-450 | 2 | |||
| 351-400 | 4 | 1 | 2 | |
| 301-350 | 3 | |||
| 251-300 | 5 | 3 | ||
| 201-250 | 3 | 3 | 1 | 9 |
| 151-200 | 4 | 4 | 9 | |
| 101-150 | 2 | 16 | 2 | 18 |
| 51-100 | 34 | 31 | ||
| 50 > | 101 | 116 | ||
Arteries embolized with NBCA-Lp were dilated and NBCA-Lp was identified as a bubble-like space containing a red cell thrombus in its internal channel. The appearance of vessel wall adjacent to the bubble-like space was characterized by stripping of endothelial cells and infiltration of inflammatory cells into the vessel wall; the median membrane was thinned or totally replaced by inflammatory cells (Figure 3B). GSPs were identified as a reticular amorphous substance with a blue-purple color on HE staining. Accumulation of inflammatory cells and red cell thrombus formation were observed around GSPs and the interstices. The appearance of vessel wall adjacent to GSPs was characterized by disappearance of endothelial cells and infiltration of inflammatory cells (Figure 3C).
Boushy et al[18] have described that paraplegia occurred with a frequency of 60% following BAE with particles (29-200 μm), and the smaller particles induced paraplegia with a higher frequency in a canine model. The occurrence of paraplegia is related to anastomosis because of communication between the anterior spinal artery and the intercostal arteries. Liebow et al[19] have documented that the left or right bronchial artery in their canine models invariably came from the right intercostal artery. Meanwhile, no paraplegia occurred in our present swine model. All animals had a common trunk of the right and left bronchial arteries without anastomosis to the intercostal artery. A common tract bronchial artery is reported generally to divide from the ventral surface of thoracic aorta with least interaction with the intercostal arteries[20,21]. Then, the different frequency of paraplegia following BAE between the two species appears to depend on the different branching style of the bronchial artery. In clinical situations, when the bronchial arteries branch from the common tract with the intercostal arteries, selective catheterization of the bronchial artery is crucial.
In both the NBCA-Lp and GSP groups, occlusion of the bronchial artery was confirmed by angiography immediately after BAE. In the GSP group, angiography 2 d after revealed partial recanalization of the bronchial arteries, whereas in the NBCA-Lp group, catheterization of the bronchial artery was difficult because of severe stenosis or complete occlusion. Occlusion was confirmed by CT as accumulation of NBCA-Lp in the principal branch artery. The duration of occlusion of the bronchial artery was greater in the NBCA-Lp group than GSP group. In histological specimens, the presence of NBCA-Lp was fulfilled as a cast, whereas GSPs were dispersed in the vessels. These results support the clinical findings that the frequency of recurrent hemoptysis was 23%-33% after embolization with GSPs[8,22] and/or PVA particles, and 10%-16.6% after NBCA-Lp embolization[8,14].
Histological examination of the lungs revealed no pulmonary infarction. The embolic materials of NBCA-Lp and GSPs were not observed in the pulmonary artery and pulmonary vein but in the bronchial branch arteries whose diameters were > 100 μm. Most rich bronchial-pulmonary artery anastomoses < 50 μm in diameter are reported to exist in the walls of the respiratory bronchioles and to supply the parenchyma of the lung[19,23]. NBCA-Lp and GSPs did not reach these anastomoses, and the circulation of the pulmonary parenchyma following BAE must have come from the pulmonary arterial circulation, resulting in no damage to the pulmonary parenchyma.
With regard to bronchial wall necrosis, according to the report of Boushy et al[18], all dogs that underwent BAE with glass microspheres (29 μm or 62 μm) caused bronchial wall necrosis. Ivanick et al[8] have reported that embolic materials < 350 μm should not be used because Pump[23] has reported two types bronchopulmonary anastomoses of 24-48 μm and 72-325 μm. Ikoma et al[24] have reported that histological examination of the lungs following BAE with NBCA-Lp in humans revealed no damage to the bronchial wall but they did not clarify the safety basis. Our present histological examination revealed that NBCA-Lp and GSPs were found in the bronchial branch arteries < 350 μm in diameter with a frequency of 53% (9/17) and 50% (3/6), respectively. Then, despite both embolic materials reaching the bronchial arteries < 350 μm in diameter, no damage to the bronchial wall was found. The bronchial walls comprise endothelial, mucosal, submucosal, smooth muscle, bronchial cartilage and adventitial layers. The bronchial arteries are distributed from the adventitia to the submucosal layer and supply blood to the bronchial wall. NBCA-Lp and GSPs were found in the bronchial arteries situated outside the bronchial cartilage but not in the fine vessels in the submucosal and the cartilage layers, because the caliber of the fine vessels was too small for NBCA-Lp or GSPs to traverse. According to Pump[23,25], bronchial arteries supply the numerous fine vessels (arterioles) to the bronchi and form a rich collateral circulation with other systemic arteries present in the esophagus, pericardium and mediastinal pleura. BAE with NBCA-Lp or GSPs resulted in occlusion of the bronchial branch artery present at the adventitia, but patency was retained in the fine vessels near the internal membrane of the bronchial wall, appearing to induce no significant ischemic damage to the bronchial wall. In the clinical situation, liquid embolic material at a ratio of NBCA 1 to Lipiodol of 1 to 7 or less for BAE is considered to be least harmful.
The present study had some limitations, which is restricted to normal lung in swine but not to hemoptysis in either normal or inflammatory lung. The degree of damage of the pulmonary parenchyma and the bronchus following embolization may be greater or less in the deviation between the swine model and clinical model for patients. A further clinical study using two embolic materials is mandatory. In addition, we did not consider the negative effect of a time elapse longer than 2 d.
In conclusion, although limitations exist, NBCA embolization causes more prolonged occlusion of the bronchial artery than GSP embolization. NBCA and Lipiodol at a ratio of 1 to 7 and GSPs were present in bronchial branch arteries ≥ 100 μm in diameter, inducing no significant harm to the bronchial wall and pulmonary parenchyma.
Bronchial artery embolization (BAE) with n-butyl cyanoacrylate (NBCA) instead of particles is reported to enhance the potency of the occlusion, aiming to reduce the incidence of recurrent hemoptysis, but it is anticipated that peripheral occlusion with higher potency BAE causes damage to the bronchial wall and pulmonary parenchyma.
The embolic materials of NBCA-lipiodol and gelatin sponge particles (GSPs) were not observed in the pulmonary artery and pulmonary vein but in the bronchial branch arteries whose diameters were > 100 μm. Most rich bronchial-pulmonary artery anastomoses < 50 μm in diameter are reported to exist in the walls of the respiratory bronchioles and to supply the parenchyma of the lung. NBCA-lipiodol and GSPs did not reach these anastomoses.
In the clinical situation based on this experimental study, liquid embolic material at a ratio of NBCA 1 to lipiodol of 1 to 7 or less for BAE is considered to be least harmful.
This is a good experimental study which clarified that NBCA owned the more potency of occlusion than GSPs, and the both NBCA and GSP were present in the bronchial branch arteries of 100 μm or greater, indicating the safety of bronchial wall and pulmonary parenchyma after BAE.
Peer reviewer: James Chow, PhD, Radiation Physicist, Radiation Medicine Program, Princess Margaret Hospital, 610 University Avenue, Toronto, ON M5G 2M9, Canada
S- Editor Cheng JX L- Editor Kerr C E- Editor Xiong L
| 1. | Rémy J, Voisin C, Dupuis C, Beguery P, Tonnel AB, Denies JL, Douay B. [Treatment of hemoptysis by embolization of the systemic circulation]. Ann Radiol (Paris). 1974;17:5-16. [PubMed] [Cited in This Article: ] |
| 2. | Uflacker R, Kaemmerer A, Picon PD, Rizzon CF, Neves CM, Oliveira ES, Oliveira ME, Azevedo SN, Ossanai R. Bronchial artery embolization in the management of hemoptysis: technical aspects and long-term results. Radiology. 1985;157:637-644. [PubMed] [Cited in This Article: ] |
| 3. | Katoh O, Kishikawa T, Yamada H, Matsumoto S, Kudo S. Recurrent bleeding after arterial embolization in patients with hemoptysis. Chest. 1990;97:541-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 79] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Hayakawa K, Tanaka F, Torizuka T, Mitsumori M, Okuno Y, Matsui A, Satoh Y, Fujiwara K, Misaki T. Bronchial artery embolization for hemoptysis: immediate and long-term results. Cardiovasc Intervent Radiol. 1992;15:154-158; discussion 158-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 97] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Cremaschi P, Nascimbene C, Vitulo P, Catanese C, Rota L, Barazzoni GC, Cornalba GP. Therapeutic embolization of bronchial artery: a successful treatment in 209 cases of relapse hemoptysis. Angiology. 1993;44:295-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Ramakantan R, Bandekar VG, Gandhi MS, Aulakh BG, Deshmukh HL. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology. 1996;200:691-694. [PubMed] [Cited in This Article: ] |
| 7. | Mal H, Rullon I, Mellot F, Brugière O, Sleiman C, Menu Y, Fournier M. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest. 1999;115:996-1001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 203] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Ivanick MJ, Thorwarth W, Donohue J, Mandell V, Delany D, Jaques PF. Infarction of the left main-stem bronchus: a complication of bronchial artery embolization. AJR Am J Roentgenol. 1983;141:535-537. [PubMed] [Cited in This Article: ] |
| 9. | Razavi MK, Murphy K. Embolization of bronchial arteries with N-butyl cyanoacrylate for management of massive hemoptysis: a technical review. Tech Vasc Interv Radiol. 2007;10:276-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 10. | Yoo DH, Yoon CJ, Kang SG, Burke CT, Lee JH, Lee CT. Bronchial and nonbronchial systemic artery embolization in patients with major hemoptysis: safety and efficacy of N-butyl cyanoacrylate. AJR Am J Roentgenol. 2011;196:W199-W204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Schenker MP, Duszak R, Soulen MC, Smith KP, Baum RA, Cope C, Freiman DB, Roberts DA, Shlansky-Goldberg RD. Upper gastrointestinal hemorrhage and transcatheter embolotherapy: clinical and technical factors impacting success and survival. J Vasc Interv Radiol. 2001;12:1263-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Kish JW, Katz MD, Marx MV, Harrell DS, Hanks SE. N-butyl cyanoacrylate embolization for control of acute arterial hemorrhage. J Vasc Interv Radiol. 2004;15:689-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Sanchez MJ, Ananian CL, Berkmen T. Embolization of an aortic arch pseudoaneurysm with coils and N-butyl-cyanoacrylate. J Vasc Interv Radiol. 2006;17:1677-1679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Baltacioğlu F, Cimşit NC, Bostanci K, Yüksel M, Kodalli N. Transarterial microcatheter glue embolization of the bronchial artery for life-threatening hemoptysis: technical and clinical results. Eur J Radiol. 2010;73:380-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Yonemitsu T, Kawai N, Sato M, Tanihata H, Takasaka I, Nakai M, Minamiguchi H, Sahara S, Iwasaki Y, Shima Y. Evaluation of transcatheter arterial embolization with gelatin sponge particles, microcoils, and n-butyl cyanoacrylate for acute arterial bleeding in a coagulopathic condition. J Vasc Interv Radiol. 2009;20:1176-1187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Sanda H, Kawai N, Ikoma A, Oda H, Sahara S. Biliary tract injury and liver damages generated after transcatheter arterial chemoembolization using porous gelatin particles(Gelpart R). Jpn intervent Radiolo. 2008;23:176-181. [Cited in This Article: ] |
| 17. | Maeda N, Osuga K, Mikami K, Higashihara H, Onishi H, Nakaya Y, Tatsumi M, Hori M, Kim T, Tomoda K. Angiographic evaluation of hepatic arterial damage after transarterial chemoembolization for hepatocellular carcinoma. Radiat Med. 2008;26:206-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Boushy SF, Helgason AH, North LB. Occlusion of the bronchial arteries by glass microspheres. Am Rev Respir Dis. 1971;103:249-263. [PubMed] [Cited in This Article: ] |
| 19. | Liebow AA, HALES MR. Studies on the lung after ligation of the pulmonary artery; anatomical changes. Am J Pathol. 1950;26:177-195. [PubMed] [Cited in This Article: ] |
| 20. | Cauldwell EW, Siekert RG. The bronchial arteries; an anatomic study of 150 human cadavers. Surg Gynecol Obstet. 1948;86:395-412. [PubMed] [Cited in This Article: ] |
| 21. | Ziyawudong J, Kawai N, Sato M, Ikoma A, Sanda H, Takeuchi T, Minamiguchi H, Nakai M, Tanaka T, Sonomura T. Aortic ostia of the bronchial arteries and tracheal bifurcation: MDCT analysis. World J Radiol. 2012;4:29-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 22. | Osaki S, Nakanishi Y, Wataya H, Takayama K, Inoue K, Takaki Y, Murayama S, Hara N. Prognosis of bronchial artery embolization in the management of hemoptysis. Respiration. 2000;67:412-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Pump KK. Distribution of bronchial arteries in the human lung. Chest. 1972;62:447-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 113] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Ikoma A, Kawai N, Sato M, Tanaka T, Sonomura T, Sahara S, Nakata K, Takasaka I, Minamiguchi H, Nakai M. Pathologic evaluation of damage to bronchial artery, bronchial wall, and pulmonary parenchyma after bronchial artery embolization with N-butyl cyanoacrylate for massive hemoptysis. J Vasc Interv Radiol. 2011;22:1212-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Pump KK. The bronchial arteries and their anastomoses in the human lung. Dis Chest. 1963;43:245-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |