INTRODUCTION
Nasopharyngeal carcinoma (NPC) is an uncommon cancer but pockets of high prevalence are found in regions of the world such as southern China, North Africa, Greenland and Alaska. In the Chinese population, the tumor is usually a non-keratinizing undifferentiated carcinoma (Type III) with a peak age incidence of 40-70 years, and is the form of the disease described in this review. Undifferentiated NPC is linked to infection with the Epstein Bar virus and has additional risk factors including diet and a genetic predisposition in family members. Because the nasopharynx is a relatively clinically silent region, patients often do not present until late when the tumor has spread into the deep tissues or to nodes in the neck. Nevertheless, this tumor is highly responsive to radiotherapy and the overall 5 years progression-free and cancer specific survival are 63% and 80%, respectively[1]. The diagnosis of NPC is made by endoscopy and confirmed on endoscopically-guided biopsy before the patient is referred for imaging to stage the cancer and plan treatment.
Head and neck computed tomography (CT) and magnetic resonance imaging (MRI) are used to stage the primary and nodal NPC, but the latter is preferred[2-5] because it is superior in terms of delineating small anatomical structures that make up the boundary of the nasopharynx, mapping tumor extent in the skull base, paranasal sinuses and brain, and discriminating between the primary tumor and adjacent retropharyngeal nodes. In some centers, fluorodeoxyglucose positron emission tomography integrated with CT (PET/CT) is advocated for NPC staging, because it may increase the accuracy of the assessment of cervical nodal metastases as well as replacing other conventional imaging techniques which are used to screen for metastases at distant sites outside the head and neck[6]. However, in these circumstances PET/CT is performed as an additional imaging modality to head and neck MRI, because MRI is superior for assessing primary tumor extent and retropharyngeal nodes[6,7].
The MRI scan should cover the head and neck from just above the skull base to just below the suprasternal notch. The protocol will vary between centers but in general T2-weighted sequences are performed in the coronal plane and axial planes, the latter with fat saturation, together with T1-weighted images in the axial +/- sagittal planes. Following a bolus injection of intravenous contrast, T1-weighted post-contrast scans are performed in at least two planes, and these should include at least one sequence without fat saturation using a 512 matrix, and one with fat saturation.
This review of staging NPC using MRI will follow the TNM staging system according to the latest 7th edition of the International Union Against Cancer/American Joint Committee on Cancer (UICC/AJCC) Cancer staging manual published in 2009/2010[8,9] which stages the primary tumor from stage T0 to T4 and the nodal metastases from N0 to N3.
MRI STAGING OF THE PRIMARY TUMOUR (T-STAGE)
Stage T0 and T1 disease
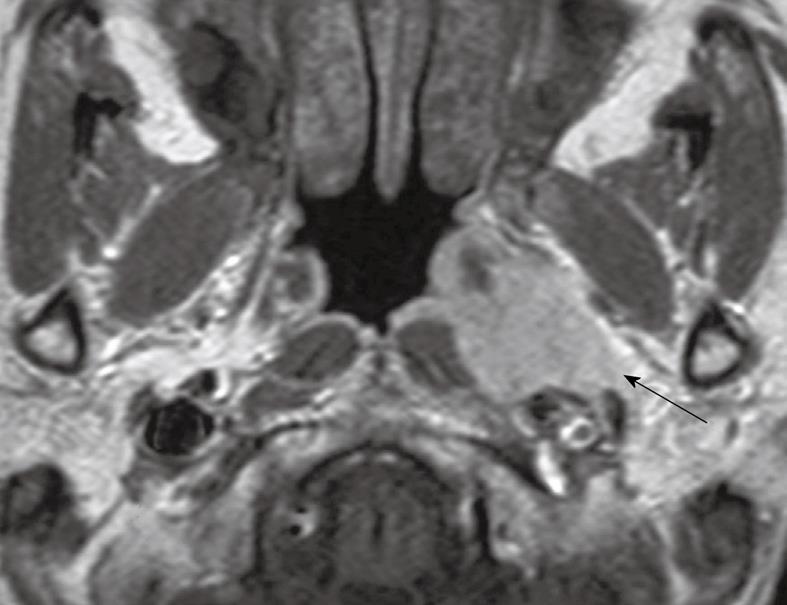
Tumor confined to the nasopharynx: The nasopharynx consists of a roof, posterior wall, lateral walls and inferior wall formed by the palate. MRI is a very sensitive technique for identifying NPC, therefore it is rare to find patients in whom the primary tumor cannot be identified (stage T0)[10]. In addition, the recent widespread use of serological testing in high risk patients with a family history of NPC is leading to the early detection of tumors[11], some of which can be clearly depicted by MRI but may be missed by endoscopy or endoscopic guided biopsy, because they are submucosal or buried deep in the lateral pharyngeal recess[10]. The latter site, also known as the fossa of Rosenmuller, lies posterior to the opening of the eustachian tube and is the most common site for NPC to arise (Figure 1)[10,12]. Mucosal spread of primary tumors tends to involve the superior portion of the nasopharynx rather than the inferior portion and palatal wall. Deep infiltrating tumors may be found even when the nasopharyngeal component is small, while other primary tumors may reach a large size and fill the nasopharyngeal cavity without extending outside the nasopharynx. However, in the non-serology screening setting, most NPCs have already spread to adjacent sites at diagnosis and so disease confined to the nasopharynx (stage T1) is only found in about a fifth of patients[13].
Figure 1 Axial post-contrast T1 weighted magnetic resonance imaging (MRI) showing a small nasopharyngeal carcinoma within the right lateral pharyngeal recess (arrow).
This is a frequent site for early cancer.
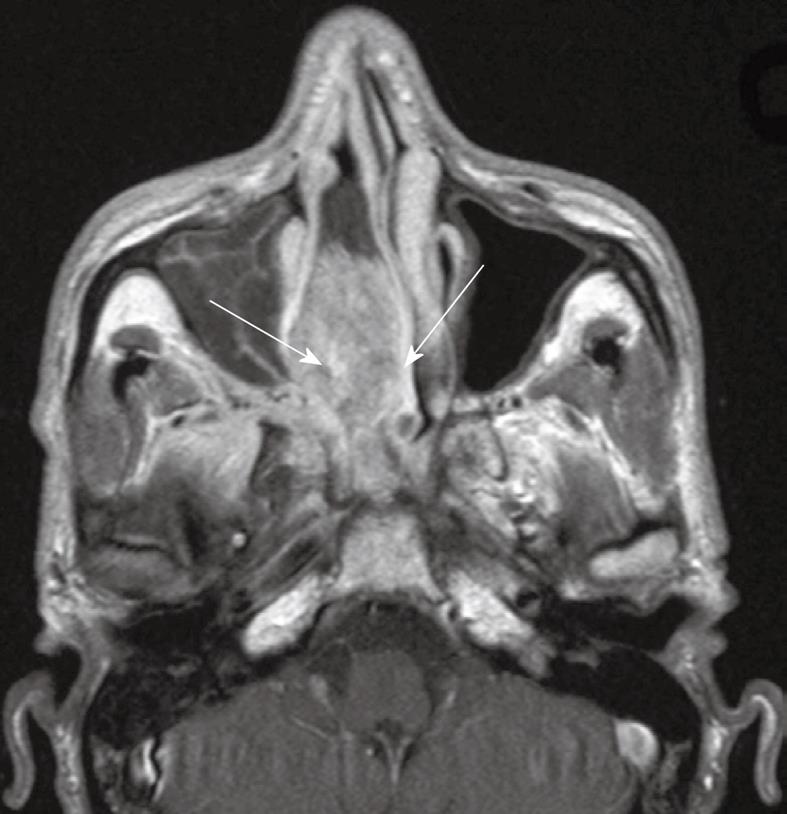
Tumor spread to the nasal cavity and oropharynx: In the latest edition of the UICC/AJCC classification, superficial spread to the nasal cavity and oropharynx has been down staged from T2 to T1 disease. The nasal cavity is commonly involved by this carcinoma because it lies directly anterior to the nasopharynx. Minimal invasion with tumor just crossing the margin of the choanal orifice is common, while more bulky disease extending into the main body of the nasal cavity is encountered less frequently (Figure 2). The nasal septum should always be scrutinized on the axial and coronal images as tumor in the nasopharyngeal roof may spread centrally along the septum.
Figure 2 Axial post-contrast T1 weighted MRI showing a bulky nasopharyngeal carcinoma with gross extension into the right nasal cavity (arrows).
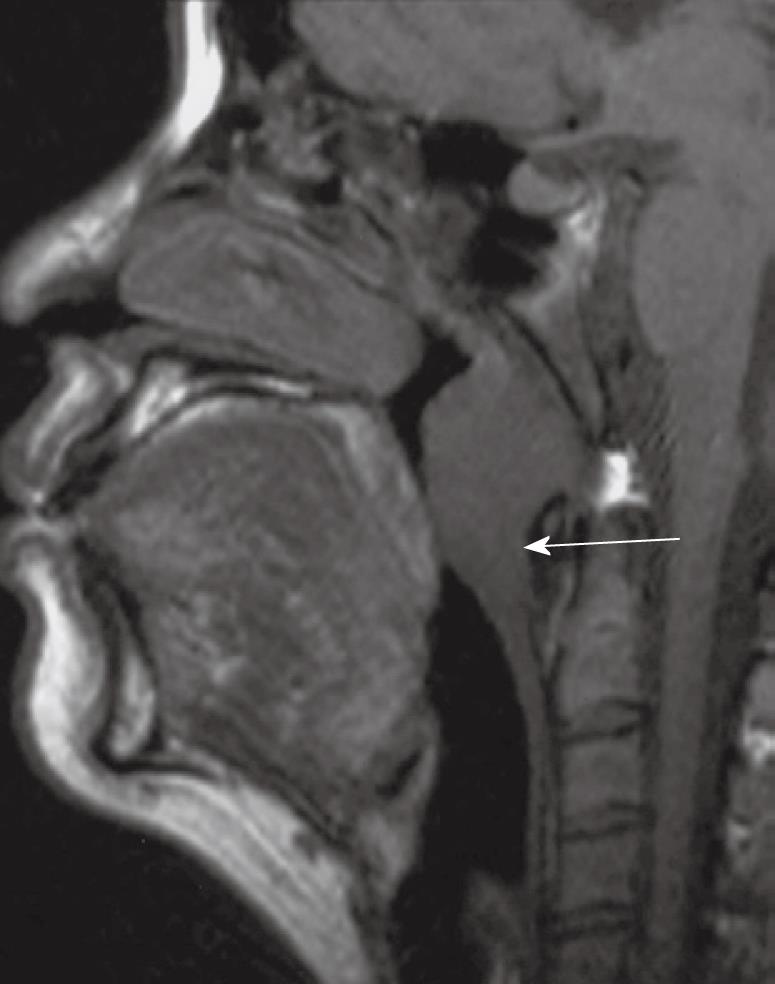
Inferior superficial extension down to the mucosa of the oropharynx is uncommon, tumors preferentially spreading superiorly to the skull base. Therefore, invasion of the oropharynx rarely occurs as an isolated event and is not usually an early sign of disease because it is already associated with tumor spread to sites such as the parapharyngeal region, skull base and cranium[13,14] (Figure 3).
Figure 3 Sagittal T1 weighted MRI showing a bulky nasopharyngeal carcinoma with inferior extension, crossing the C1/2 level, along the posterior wall into the oropharynx (arrow).
Stage T2 disease
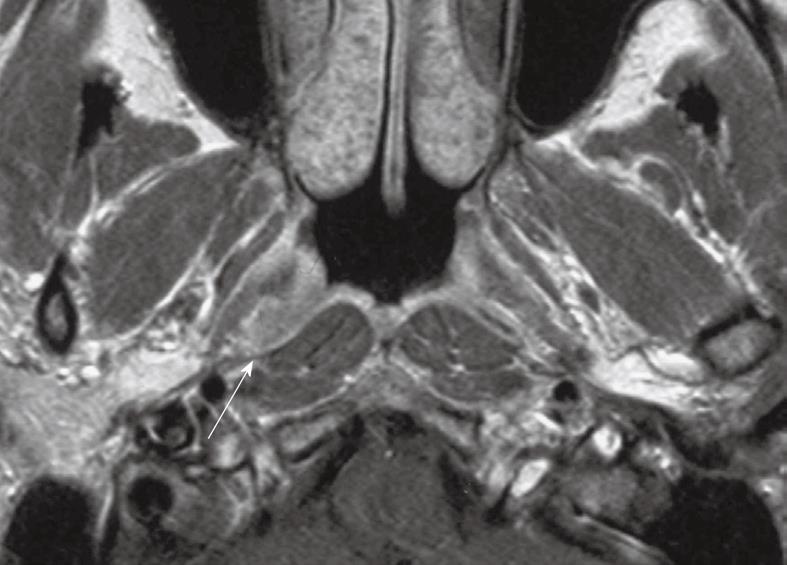
Tumor spread to the parapharynx: Deep extension into the parapharyngeal region has been changed from stage T2b to stage T2. Parapharyngeal spread occurs when tumor spreads posterolaterally from the nasopharynx and usually involves lateral penetration through the levator palatini muscle and pharyngobasilar fascia to involve the tensor palatini muscle and parapharyngeal fat space which contains the pharyngeal venous plexus. Parapharyngeal invasion is associated with an increased risk of distant metastases, tumor recurrence and survival[15-17]. MRI is able to distinguish between a primary tumor confined to the nasopharynx that is only bulging into the fat space (stage T1), a primary tumor confined to the nasopharynx which is abutting a metastatic retropharyngeal node (stage T1N1), and a primary tumor that is directly invading the parapharyngeal region (stage T2) (Figure 4)[18]. Parapharyngeal involvement can lead to compression of the eustachian tube resulting in a middle ear and mastoid effusion. Further posterolateral spread may also involve the carotid space and encase the carotid artery.
Figure 4 Axial post-contrast T1 weighted MRI showing a nasopharyngeal carcinoma directly infiltrating into the left parapharyngeal space (arrow).
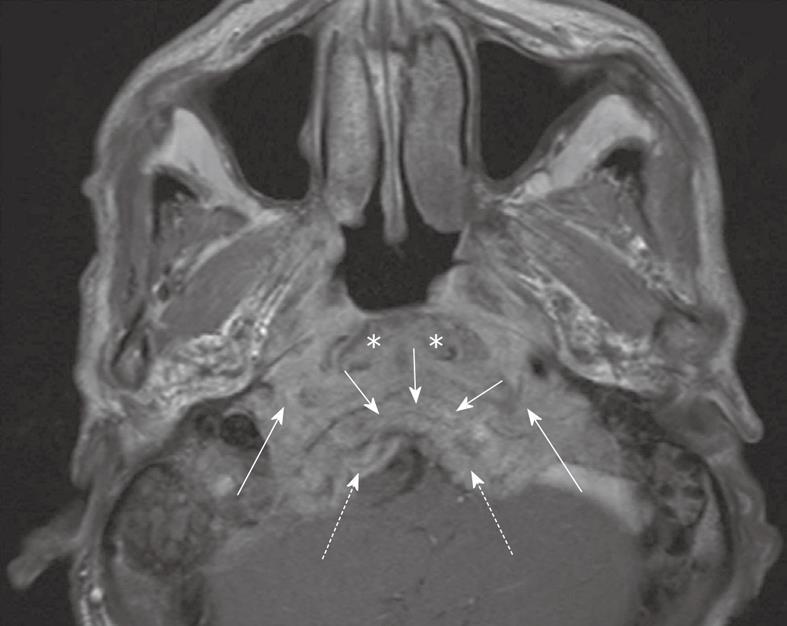
Directly posterior to the nasopharynx is the retropharyngeal region, longus capitis muscles, prevertebral space and clivus (Figure 5). Direct posterior extension into these sites may be the only site of invasion outside the nasopharynx. In some patients this posterior extension is the preferred pattern of tumor spread with bulky disease continuing down to the foramen magnum and upper cervical spine. This region contains lymphatics and a venous plexus and so invasion of the prevertebral space is associated with an increased risk of distant metastases and decreased survival[19].
Figure 5 Axial post-contrast T1-weighted MRI showing a nasopharyngeal carcinoma with gross posterior retropharyngeal extension in the longus capitis muscles (asterisks), prevertebral space (long arrows), clivus (short arrows) and posterior cranial fossa (broken arrows).
Stage T3 disease
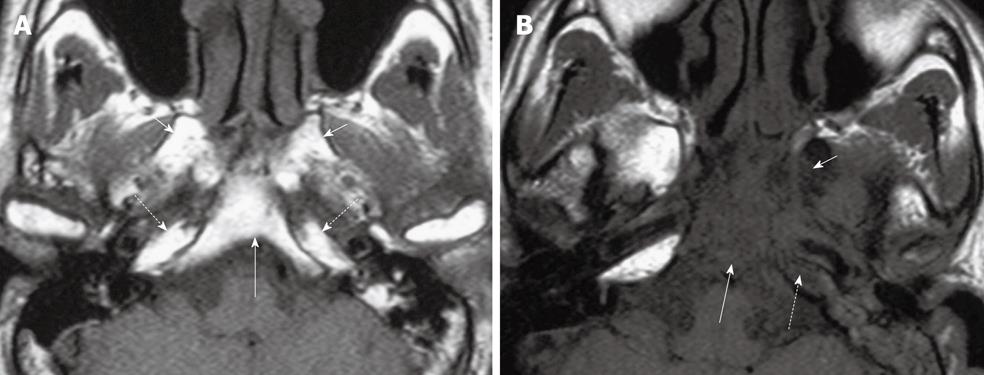
Tumor spread to the skull base: Tumor invasion into the skull base or paranasal sinuses remains stage T3 disease in the latest edition of the UICC/AJCC classification. NPC has a propensity to invade bone and over 60% of patients have skull base invasion at diagnosis[13]. The number of bony sites may influence prognosis[20] and can vary from extensive invasion involving multiple sites to only a small localized area, which in some patients may be the only site of extra nasopharyngeal spread. The clivus, pterygoid bones, body of the sphenoid and apices of the petrous temporal bones are most commonly invaded. The axial T1 weighted image provides a good overview of the extent of any skull base invasion and interrogation relies on identifying five key regions of high T1 signal fatty bone marrow which comprise the clivus; right pterygoid base; left pterygoid base; right petrous apex; left petrous apex (Figure 6A and B). The body of the sphenoid is more difficult to assess because it forms a thin shelf around the sphenoid sinus but can be studied on the coronal images. After identification of the major sites of bony invasion, other sites including the sphenoid wings and upper cervical spine should be assessed before scrutinizing the skull base foramina and fissures. The skull base foramina form an unimpeded channel for tumor spread, but there is often direct invasion of the bones bordering these foramina. The foramina are assessed best on the coronal images, and passing from the anterior to the posterior skull base they consist of the foramen rotundum (V2 nerve) and vidian canal (vidian nerve) (Figure 7A); the foramen ovale (V3 nerve) (Figure 7B); and foramen lacerum (which lies below the horizontal portion of the internal carotid artery) (Figure 7C). The foramen ovale and lacerum are two commonly involved foramina which provide a route of tumor spread into the cranium[2,13,21].
Figure 6 Axial T1 weighted MRI of the skull base showing five key bony sites to check for tumor invasion.
A: Normal skull base showing T1W weighted signal of normal fatty bone marrow within the clivus (long arrow), bilateral pterygoid bases (short arrows) and petrous apices (broken arrows); B: Abnormal skull base showing loss of normal high T1 weighted signal due to tumor invasion of the clivus (long arrow), left pterygoid base (short arrow) and left petrous apex (broken arrow).
Figure 7 Coronal post-contrast T1 weighted MRIs of nasopharyngeal carcinoma in three patients illustrating tumor extension in the foramina from the anterior (A) to the posterior (C) skull base.
A: The right foramen rotundum and vidian canal (solid long and short arrows, respectively); B: Left foramen ovale (solid arrow); C: Left foramen lacerum (solid arrow). The uninvolved foramina on the contralateral sides are indicated by broken arrows.
Inferior spread of tumor to involve the hypoglossal nerve canal (XII nerve) and jugular foramen (IX-XI nerves) is less common, but in the case of the hypoglossal nerve canal, denervation of the hemitongue may be found.
Tumor may also involve the pterygomaxillary fissure, which lies posterior to the maxillary sinus, and the petroclival fissure. Finally, positioned in the central skull base is the pterygopalatine fossa (Figure 8) which forms a very important crossroads connecting the skull base to the face and brain. This fossa can be located in the most medial aspect of the pterygomaxillary fissure on the axial images and provides a route of tumor spread to the orbit (via the inferior orbital fissure), infratemporal fossa (via the pterygomaxillary fissure), oral cavity (via the pterygopalatine canal), nasal cavity (via the sphenopalatine foramen), foramen lacerum (via the vidian canal) and middle cranial fossa (via foramen rotundum).
Figure 8 Axial post-contrast T1 weighted MRI showing contiguous extension of nasopharyngeal carcinoma into the left pterygopalatine fossa which is expanded (long arrow).
Compare this with the normal hyperintense fat signal in the narrow pterygopalatine fossa on the contralateral side (short arrow).
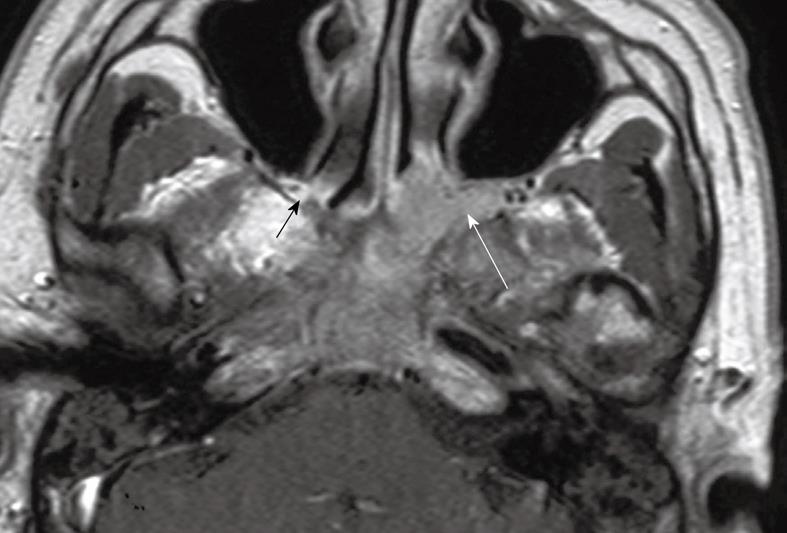
Tumor spread to the paranasal sinuses: Mucosal inflammatory changes in the paranasal sinuses are common in patients with NPC, and MRI has the advantage over CT of providing better discrimination between tumor and these benign changes. The sphenoid sinus is frequently invaded by NPC because it lies immediately above the roof of the nasopharynx from which it is separated by a thin plate of bone (Figure 9). Tumor invasion of the ethmoid sinuses usually occurs from direct spread from the sphenoid sinus or nasal cavity, and invasion at this site may reduce the chance of shielding the optic nerve from the radiotherapy field. The maxillary sinus is rarely involved except as a late event when there is usually extensive invasion elsewhere, not only within the nasal cavity, but also the sphenoid and ethmoid sinuses, skull base and brain[13].
Figure 9 Coronal post-contrast T1 weighted MRI showing a nasopharyngeal carcinoma with direct infiltration through the sphenoid body (long arrows) into the sphenoid sinus (short arrows) and right cavernous sinus (broken arrows).
Stage T4 disease
Tumor spread to the intracranium, cranial nerves and orbit: NPC invades the cavernous sinus (Figure 9) and dura[2,13], while direct invasion of the brain at diagnosis is rare. Invasion of the cavernous sinus can lead to multiple cranial nerve palsies involving cranial nerves III, IV, V1, V2 and VI. NPC may spread into the cavernous sinus from multiple directions including posteriorly from tumor surrounding the horizontal portion of the internal carotid artery, anteriorly from the orbital fissures or through the skull base in the region of the foramen ovale or sphenoid sinus. Dural invasion usually involves the floor of the middle cranial fossa adjacent to the cavernous sinus and foramen ovale, while posterior fossa invasion occurs along the posterior aspect of the clivus and occasionally along the tentorium.
Cranial nerve involvement is a clinical rather than radiological sign for staging, although tumor may be seen on MRI on the post-contrast T1-weighted images with fat saturation, especially around the V3 and V2 nerves in the foramen ovale and rotundum, respectively. True perineural spread, in which tumor extends a long distance along the nerve away from the primary tumor site and foramina in the skull base, is occasionally found, but in general is uncommon in the pre-treatment setting. Orbital invasion is also a marker of the most extensive form of NPC and is usually invaded by tumor in the pterygopalatine fossa travelling via the inferior orbital fissure or directly from the cavernous sinus.
Tumor spread to the infratemporal fossa/masticator space and hypopharynx: Invasion of the medial and lateral pterygoid muscles, infratemporal fat and temporalis muscle is denoted as T4 disease and is usually found when tumors extend laterally from the parapharyngeal space, pterygoid base or the pterygomaxillary fissure. The hypopharynx is the most inferior site of tumor invasion included in the staging classification, but it is very rarely involved at diagnosis because, as noted above, NPC has a preference for extending superiorly to the skull base rather than inferiorly to the oropharynx and then hypopharynx.
MRI STAGING OF NODAL METASTASES (N-STAGE)
Patterns of nodal spread
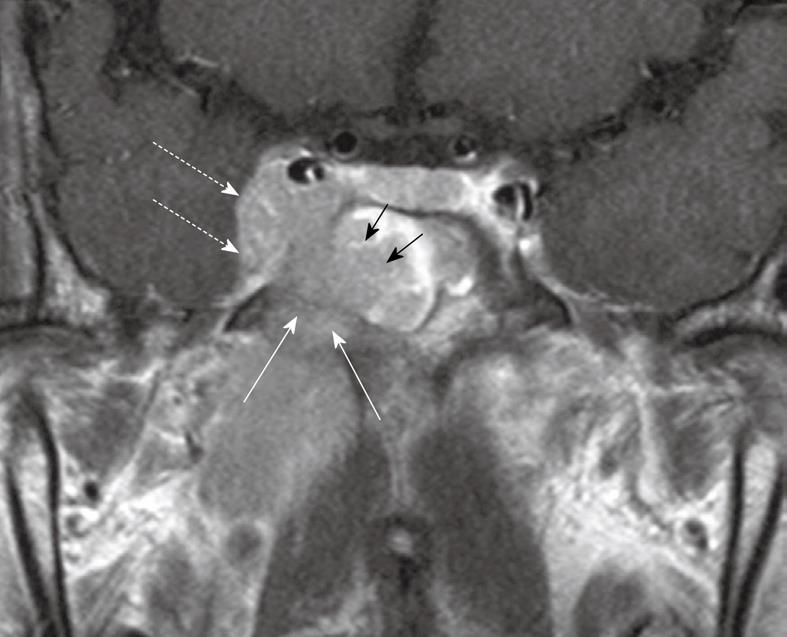
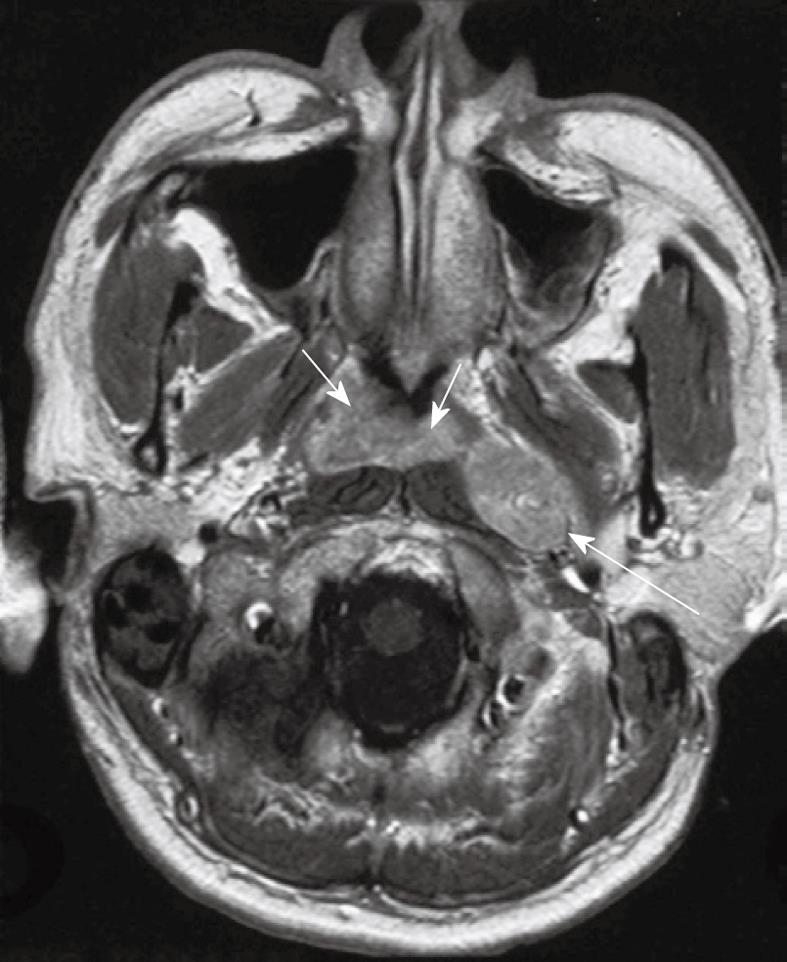
NPC has a propensity to spread to nodes with over 75% of patients having nodal metastases at presentation and enlarged nodes may be the first clinical manifestation of disease. Nodal metastases are not necessarily related to the size[22] or stage of the primary tumor because patients with small tumors may have extensive nodal metastases while some bulky tumors invading the skull base show no nodal spread. Nodal metastases from NPC have a tendency for bilateral neck spread. Lateral retropharyngeal nodes, which lie medial to the carotid artery (Figure 10), are one of the most common sites of nodal spread from NPC[23] and are easily identified on MRI[24,25] and as such have been considered the first echelon of metastatic spread[23,25]. However, it has now been shown that metastatic nodal spread may bypass these nodes and spread directly to the non-retropharyngeal nodes in the upper neck, usually to the level II nodes[26-29]. It should also be noted that the metastatic lateral retropharyngeal nodes can be identified on MRI from the skull base to the level of C3[25], and therefore they frequently extend deep to the oropharyngeal wall (Figure 11). The median group of retropharyngeal nodes does not form a discrete nodal chain[30] and as such are not usually identified on MRI in NPC[24,25].
Figure 10 Axial post-contrast T1 weighted MRI showing a nasopharyngeal carcinoma (short arrows) and a grossly enlarged metastatic left retropharyngeal lymph node at the level of the nasopharynx (long arrow).
Figure 11 Axial post-contrast T1 weighted MRI in a patient with nasopharyngeal carcinoma showing an enlarged left sided metastatic retropharyngeal node deep to the oropharynx (long arrow).
An enlarged metastatic left upper internal jugular node posterior to the left internal jugular vein (level IIB) is also present which is a very common site of nodal metastases (short arrow).
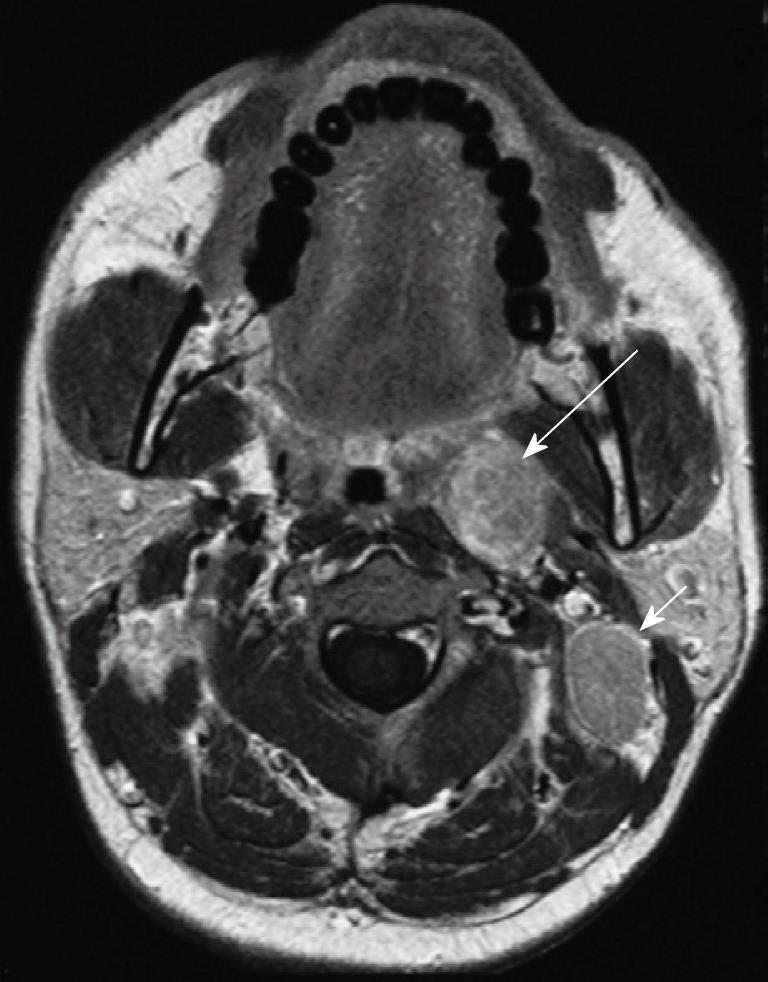
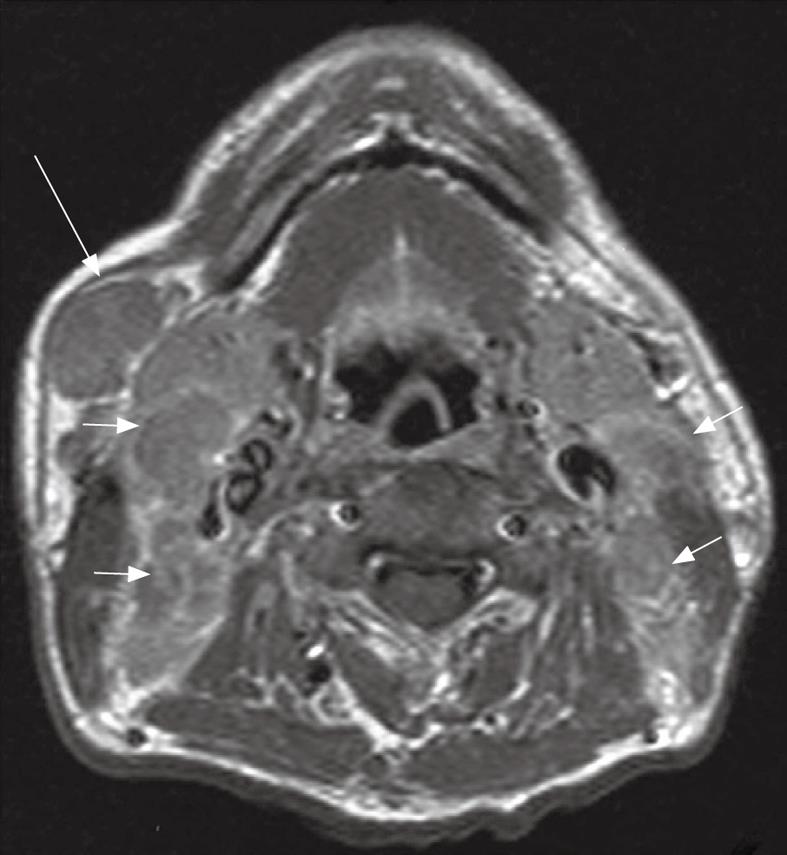
Skip metastases to the lower neck have been described[29] but usually nodal metastases spread in an orderly fashion[31] down the neck involving the nodal groups along the internal jugular chain (level II to IV), and spinal accessory chain in the posterior triangle (Va and Vb). Metastatic nodes posterior to the jugular vein in the upper neck (Figure 11) are the most common site for non-retropharyngeal nodes[27] and are designated as high internal jugular nodes (level IIa or b), although at this site the internal jugular and spinal accessory nodal chains converge. Nodes in the submandibular and parotid/periparotid region are far less common at diagnosis but should be searched for because of the implications for planning radiotherapy especially intensity modulated radiotherapy. Nodes at these sites may be found when there are bulky upper cervical nodes (Figure 12) which obstruct the normal routes of lymphatic drainage[25,29]. Once the nodal metastases reach the supraclavicular fossa there is an increased incidence of distant metastases.
Figure 12 Axial post-contrast T1 weighted MRI in a patient with nasopharyngeal carcinoma showing bulky metastatic nodes in the internal jugular chains (short arrows) and right submandibular region (long arrow).
Diagnosis of nodal metastases
MRI diagnoses nodal metastases on the basis of size if the shortest nodal axial diameter reaches 5 mm or greater in the lateral retropharyngeal region[25,32], 11 mm in the jugulodigastric region or 10 mm in other non-retropharyngeal nodes of the neck, or if there are a group of three or more nodes which are borderline in size[33]. However, it should be noted that normal nodes become progressively smaller moving caudally in the neck and therefore a size of 5-7 mm is sometimes used as a cut-off in the lower neck. NPC nodes are often necrotic and show extracapsular spread and these signs are used by MRI to identify metastatic nodes irrespective of size[33,34]. Extracapsular spread has also been shown to be an independent prognostic factor for overall survival and distant metastases failure-free survival[35].
Staging
Staging of nodal metastases from NPC differs from that of other carcinomas in the head and neck. The most recent 7th edition UICC/AJCC classification includes the retropharyngeal nodes which have been shown to have a negative effect on prognosis[36], and is classified as N1 irrespective of whether they are unilateral or bilateral. All other cervical nodes are considered to be stage N1 if unilateral and stage N2 if bilateral, unless they are greater than 6 cm in size or reach the supraclavicular fossa, in which case they are designated as stage N3a or N3b, respectively. Unlike other carcinomas in the neck, N2 is not further divided into substages according to the number of nodes or bilateral/contralateral involvement. Matted nodes forming a nodal mass of greater than 6 cm is rare and therefore most patients with stage N3 are diagnosed on the basis of supraclavicular nodes.
MRI STAGING OF DISTANT METASTASES IN THE HEAD AND NECK (M-STAGE)
Distant metastases are found in about 5% of patients[37] at diagnosis and are most frequently found in the skeletal system followed by the thorax (lymph nodes and lungs) and then the liver. Although distant metastases are uncommon at diagnosis, head and neck MRI studies should always be assessed for evidence of spread to the bones in this region or lung apices, especially in patients with risk factors such as metastatic cervical nodes which extend to the supraclavicular fossa.
CONCLUSION
Head and neck MRI is the best modality for staging locoregional NPC, and the common sites for local primary tumor invasion and patterns of nodal spread have been described. Recent minor changes to the latest 7th edition of the UICC/AJCC cancer staging manual have seen primary tumor spread to the nasal cavity and oropharynx down staged from stage T2A to stage T1, and parapharyngeal spread changed from stage T2a to T2, while for nodal staging the retropharyngeal nodes have now been incorporated officially into the staging system as stage N1 disease, irrespective of whether they are unilateral or bilateral.