INTRODUCTION
Ultrasound (US) is often the first mode of examination in patients with pancreatic disease. The introduction of contrast-enhanced ultrasonography (CEUS) has led to great improvements in the diagnostic capabilities of US[1].
CEUS takes advantage of its special features: the high contrast and spatial resolution, the use of a blood-pool microbubble contrast medium and the real-time, dynamic evaluation of tumor enhancement, filtering the background tissue signals[1-3]. CEUS is a sensitive imaging method for evaluating the vascularization of pancreatic lesions both solid and cystic[2-7]. The innovative use of CEUS for pancreatic study creates the need for a definition of the most frequent dynamic features of solid and cystic masses.
To overcome subjectivity, the use of quantification software could be suggested for the characterization of pancreatic lesions during CEUS study, as recently reported in the literature[8]. Its high capability in showing tumoral microcirculation also makes CEUS accurate in the study of neoangiogenesis[9]. Interest in the use of CEUS for noninvasive prognostic stratification of pancreatic adenocarcinoma and for the evaluation of chemotherapeutic effects is documented in the literature[10-13].
CEUS is less expensive compared to computed tomography (CT) and magnetic resonance imaging (MRI) and can also be used in patients with renal failure[1,14]. CEUS is able to significantly improve the accuracy of US, allowing a better characterization and staging of pancreatic pathologies[2-7].
TECHNICAL BACKGROUND AND CONTRAST MEDIA
CEUS is the only imaging method that allows a real-time evaluation of the enhancement during the dynamic phases. Harmonic microbubble (MB)-specific imaging with a low acoustic US pressure (Mechanical index, < 0.2) is required for a dynamic CEUS examination. All the background tissue signals are filtered and the vascular enhancement signals are only related to the harmonic responses of the MBs. A 2.4 mL bolus of second-generation contrast agent, constituted by sulphur hexafluoride filled microbubbles with a phospholipid peripheral shell (SonoVue®, Bracco, Milan, Italy), is injected i.v. followed by a 5 mL bolus of saline solution. A real-time evaluation of the enhancement is possible, maintaining the same scanning frame rate as in the previous conventional B-mode examination. Dynamic observation of the contrast-enhanced phases (arterial, portal/venous and late phases) begins immediately after the injection of the microbubbles[15].
These typical features of CEUS make this method very accurate in perfusion studies, allowing the visualization of the pancreatic lesion microvasculature[2,7]. Some major limitations are the occasionally restricted image resolution of deep regions and the poor sonographic visualization of the gland due to overlying abdominal gas or to large amounts of abdominal fat[1,2].
CLINICAL APPLICATIONS
Inflammatory diseases
Acute pancreatitis: Acute focal pancreatitis, even when supported by clinical data, can cause problems of differential diagnosis in respect to neoplastic lesions[16,17]. A mild acute focal pancreatitis appears as a homogeneously hypoechoic focal enlargement of the gland in conventional US[16,17], and hypervascularized after the administration of contrast agent[17], with different degree of enhancement, resulting in an increased echogenicity in the dynamic phases.
In severe acute pancreatitis, CEUS may improve the detection and delimitation of the necrotic areas, which appear completely avascular[17]. Unfortunately, in the literature there are no studies comparing CEUS with CT or MRI in the evaluation and follow-up of acute pancreatitis. At this moment, CT remains more effective than CEUS, in particular in grading the stage of the disease[18].
Pseudocysts: Pseudocysts can be sequelae of severe acute pancreatitis or can occur in chronic pancreatitis[18]. Characterized by a fibrous wall without an epithelial lining[19], pseudocysts must be differentiated from pancreatic cystic tumors, especially mucinous cystadenomas (MCAs), as they require completely different therapeutic approaches[19]. CEUS has a crucial role in differential diagnosis of pseudocysts and pancreatic cystic tumors, by better evaluating the micro-vascularization of the intralesional inclusions. Even if characterized by a corpuscular and inhomogeneous content in conventional US, pseudocysts are always completely avascular, becoming homogeneously anechoic in CEUS dynamic examination[2].
Mass forming chronic pancreatitis: Mass-forming chronic pancreatitis usually occurs in patients with a history of chronic pancreatitis[20] and must be differentiated from pancreatic ductal adenocarcinoma[2,7,21], although they are both hypoechoic in conventional US. In CEUS, a mass-forming chronic pancreatitis shows ‘parenchymographic’ enhancement, characterized by an enhancement pattern always comparable to that of the surrounding pancreatic parenchyma. However, in long-standing chronic inflammatory processes, inhomogeneous hypovascularization of the lesion may be observed, probably owing to the presence of a large amount of fibrosis, and the differential diagnosis becomes more difficult[2,7,21].
Autoimmune pancreatitis: Autoimmune pancreatitis is a particular type of chronic pancreatitis. Characterized by periductal flogosis, mainly sustained by lymphocytic infiltration, with evolution to fibrosis, this pancreatic pathology has a recent pathological definition[22]. The US features resemble those of focal pancreatitis, even if autoimmune pancreatitis more frequently involves the entire gland, with a global enlargement of the pancreas. In all cases, in conventional US the echogenicity is typically markedly reduced and the main pancreatic duct compressed. After the administration of contrast agent, autoimmune pancreatitis shows inhomogeneous a fair, often moderate to marked, enhancement usually followed by a slow washout. These features are related to the thinning of the glandular vessels due to thick lymphocytic infiltration and fibrosis.
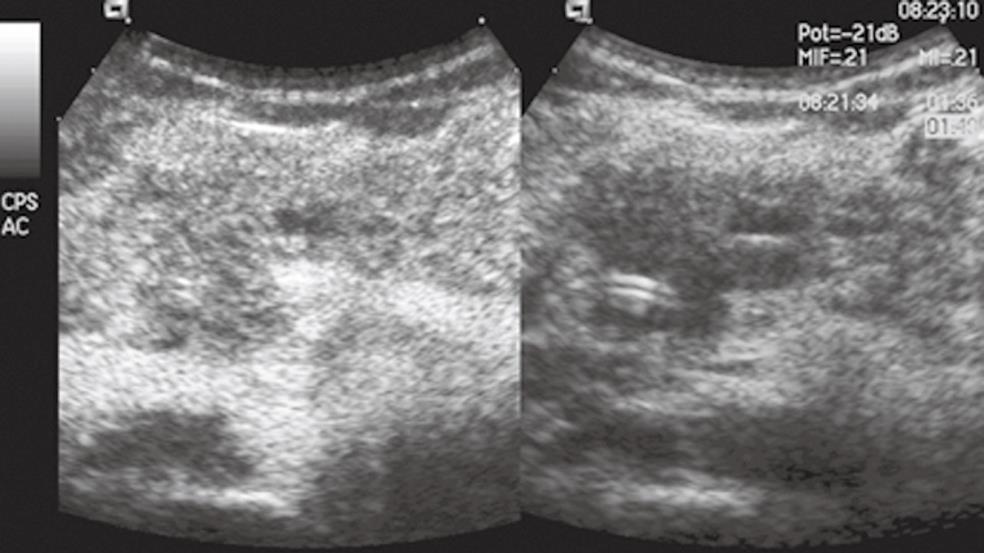
CEUS findings may be especially useful in the study of focal forms of autoimmune chronic pancreatitis (Figure 1), in which differential diagnosis in respect to ductal adenocarcinoma is a priority[21,23].
Figure 1 Focal autoimmune pancreatitis.
The pancreatic head mass is hypoechoic in conventional ultrasound (US) (right side of the split-screen) and inhomogeneously isovascular in contrast-enhanced US (CEUS) (left side of the split-screen).
Solid neoplasms
Ductal adenocarcinoma: Pancreatic tumors are classified according to their histological type and grade in the WHO classification[24]. Ductal adenocarcinoma comprises between 80% and 90% of all exocrine tumors of the pancreas. It usually presents as a solid mass with infiltrative ill-defined growth margins, typically hypoechoic in conventional US. The main pancreatic duct is usually infiltrated and dilated upstream. Doppler studies show poor or no vascularity inside the lesion and the vascular invasion is defined by a focal absence of the echogenic interface of the vessel wall, or by a narrow lumen, with changes in blood flow velocity[25-27]. In CEUS examination, ductal adenocarcinoma typically shows poor enhancement (Figure 2) during all the dynamic phases because of its intense desmoplastic reaction with a relatively poor mean vascular density and perfusion[5,6,23]. The degree of differentiation of the lesion influences its microvascular density[28]: markedly hypovascular masses with very low mean vascular density and perfusion, characterized by avascular areas due to the presence of necrosis, correspond to highly aggressive forms, un-differentiated at pathology, with the poorest prognosis[10].
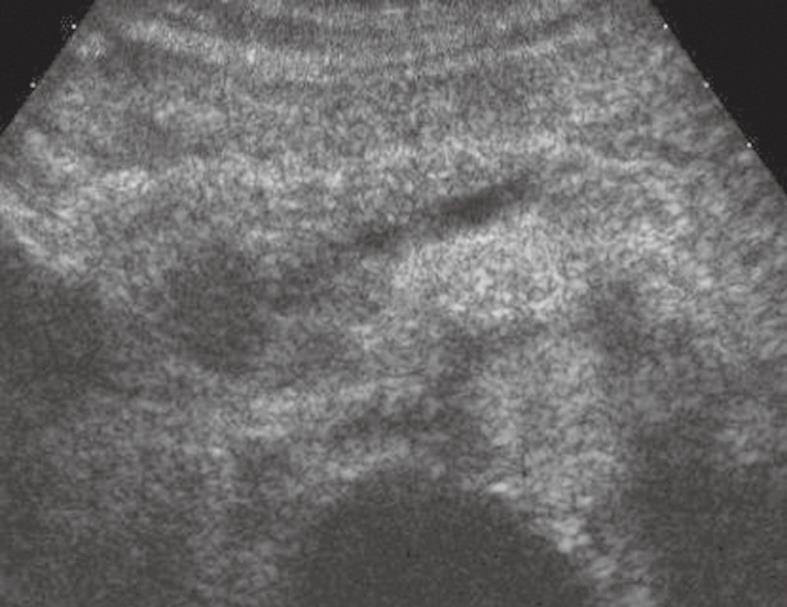
Figure 2 Pancreatic ductal adenocarcinoma.
The pancreatic head solid lesion is hypoechoic in CEUS with upstream dilation of the main pancreatic duct.
Loco-regional US staging of ductal adenocarcinoma is very accurate[20] and after the administration of microbubbles both margins and size of the lesion are more visible, improving the detection of vascular infiltration or involvement. In addition, CEUS improves hepatic staging, allowing a higher accuracy in the detection and characterization of distant metastases[18].
Endocrine tumors: Endocrine tumors arise from the neuroendocrine cells of the pancreas and may induce specific clinical syndrome related to the tumor-released hormones (clinically classified as functioning endocrine tumors), or aspecific symptoms resulting from the expansive growth and tumor size [clinically classified as nonfunctioning endocrine tumors (NFETs)][19,29]. In conventional US, they usually present as well-demarked homogeneous hypoechoic masses[30].
Imaging differentiation with ductal adenocarcinoma is fundamental for determining the therapeutic strategy and prognosis[31]. The main pancreatic duct is usually not infiltrated. In Doppler examination, small vessels characterized by arterial flow within the lesion are often detected. However, Doppler ‘‘silence’’ does not exclude the diagnosis of an endocrine tumor owing to the possible small size of the tumor vascular network[15].
In CEUS, endocrine tumors usually appear hypervascular (Figure 3). Voluminous endocrine tumors show rapid and intense enhancement during the early dynamic phases, often with avascular necrotic intralesional areas[31-33]. Rarely, NFETs can also be hypovascularized in imaging, depending on the amount of dense and hyalinized stroma inside the lesion. CEUS improves local and hepatic staging, allowing a higher accuracy in the detection and characterization of distant metastases[15,34].
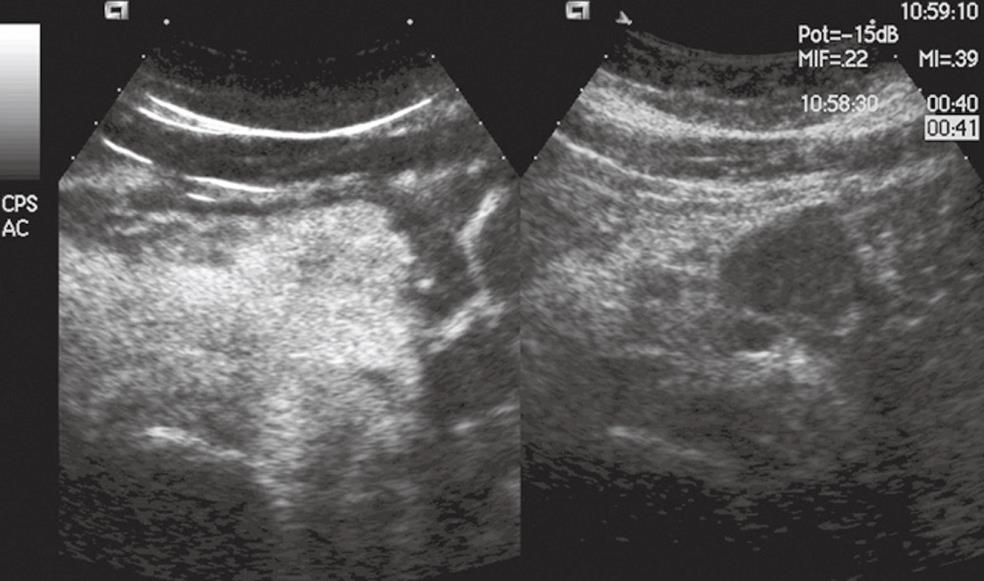
Figure 3 Pancreatic endocrine tumor.
The pancreatic body mass is solid and hypoechoic in conventional US (right side of the split-screen) and hypervascular in CEUS (left side of the split-screen).
Metastases: Pancreatic metastases are rare. The most common are from renal cell carcinoma. CEUS may well demonstrate the enhancement of pancreatic metastases from renal cell carcinoma, as they are clearly hypervascular, allowing differential diagnosis against ductal adenocarcinoma. However, their CEUS features cannot be differentiated from those of endocrine tumors. The differential diagnosis is therefore based on the clinical history and symptoms[35,36].
Cystic neoplasms
Serous cystadenoma: Serous cystadenoma (SCA) is a benign lesion[37], usually solitary, without communication with the main pancreatic duct. Typically it presents as a microcystic well-defined lesion, macroscopically characterized by multiple small cysts separated by thin septa orientated to the center[19,38,39]. In 15% of cases a central scar may be present[38]. CEUS improves the US characterization of SCA, showing the enhancement of internal septa, with better identification of the “honeycomb” multilocular architecture of the lesion[17]. The macrocystic type (25%) is divided into the mixed type with large cysts and the unilocular type difficult to be differentiated from MCA[40]. CEUS is helpful not only in the differential diagnosis of SCA, but also in the long-term follow-up of these tumors, which can be conservatively managed in most cases[3].
Mucinous cystic tumors: Mucin-producing tumors of the pancreas may originate either from the peripheral ducts (mucinous cystic tumors) or from the main pancreatic duct and its collateral branches [intraductal papillary mucinous neoplasms (IPMNs)][41].
MCA is a potentially malignant lesion[42], which may degenerate into cystadenocarcinoma[37,39,43]. Imaging characterization is therefore mandatory for a correct therapeutic approach. MCA typically presents as a single round macrocystic lesion, usually located in the body-tail of the gland, without communication with the main pancreatic duct[44,45]. Often large and multilocular, but sometimes unilocular, in conventional US, it is characterized by a dense content resulting from the presence of mucin, and irregular thick wall and internal septa. The malignant degeneration into cystadenocarcinoma is usually characterized by the evidence of parietal nodules. The administration of contrast agent is necessary for a correct diagnosis, i.e. the identification of vascularized inclusions (Figure 4), and for the differential diagnosis between MCA and a pseudocyst[17,46].
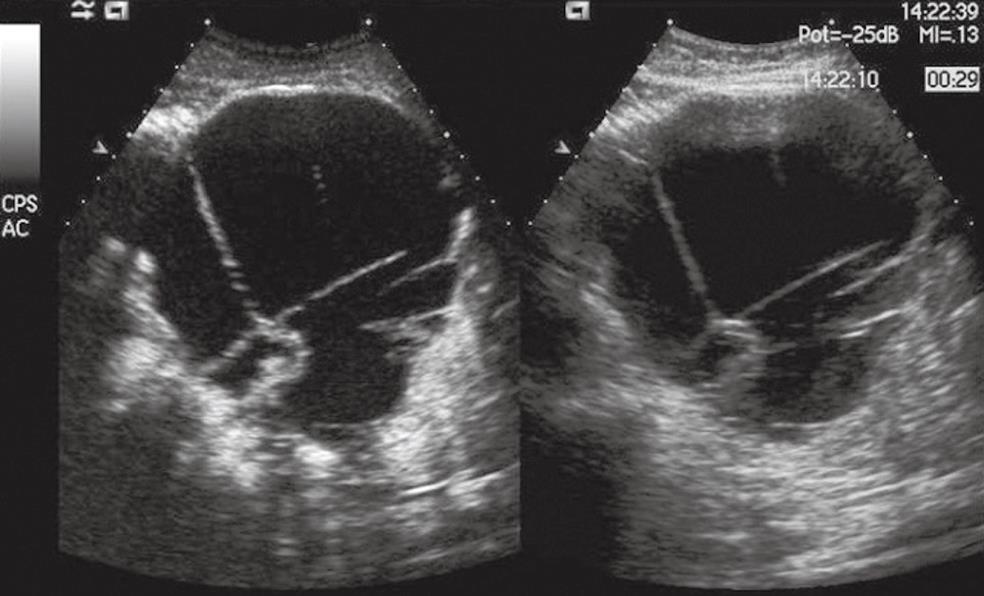
Figure 4 Pancreatic mucinous cystoadenocarcinoma.
A voluminous cystic mass is seen in the pancreatic body with septa and nodules in conventional US (right side of the split-screen) and is enhanced in CEUS (left side of the split-screen).
IPMNs are cystic tumors of the pancreas recently reported with increasing frequency[6,38,41,47,48]. They are macroscopically characterized by having an intraductal origin and growth[49], with the production of dense mucin that fills the main pancreatic duct (the ductectatic mucin-hypersecreting variant) or with endoluminal papillary proliferation (the papillary-villous variant). The involvement or demonstration of communication with the main pancreatic duct is mandatory for a correct diagnosis[50,51]. They can be divided into 3 types: the central type, with focal or diffuse dilation of the main pancreatic duct; the side branch type, characterized by unilocular or multilocular cystic lesions with grapelike clusters; and the mixed type. US usually demonstrates dilatation of the main pancreatic duct, although MRI still remains the gold standard[51]. CEUS study allows a better detection and characterization of intraductal papillary vegetation, especially in the papillary-villous variant, demonstrating their vascularization[17], thus assisting in the differentiation between benign and malignant lesions[4].
The presence of mural nodules, thick septa and a Wirsung’s duct diameter greater than 10 mm are suggestive for malignancy[52].
Solid-pseudopapillary tumor: A solid-pseudopapillary tumor (SPT) is a rare low-grade malignancy of the exocrine pancreas, typically presenting as a large well-defined round mass, without communication with the main pancreatic duct. In conventional US, it shows a inhomogeneous aspect because of hemorrhagic or necrotic or cystic degeneration[19,50]. After the administration of contrast agent, SPT typically shows inhomogeneous enhancement of the thickened peripheral capsule and solid components surrounding cystic and necrotic avascular areas.