Published online Mar 28, 2024. doi: 10.4329/wjr.v16.i3.58
Peer-review started: December 10, 2023
First decision: January 6, 2024
Revised: January 20, 2024
Accepted: March 6, 2024
Article in press: March 6, 2024
Published online: March 28, 2024
Fibroadenoma (FA) is the most common tumor found in young women, although it can occur in any age group. Ductal carcinoma in situ (DCIS) that is confined in a FA is rare; it is most frequently reported as an incidental finding.
We report a case of DCIS within a FA in a 46-year-old female without cancer-related personal and family histories. The patient was diagnosed with a breast conglomerate of nodules and was followed for 1 year. In the current control image study, we found suspicious microcalcification, as a new finding, within one of the nodules. Consequently, a core biopsy of the tumor, which appeared hypoechoic, oval, and circumscribed, was performed. The pathological diagnosis was ductal carcinoma in situ within a fibroepithelial lesion. The patient underwent breast-conserving surgery and received radiotherapy as well as endocrine therapy (tamoxifen).
We recommend a multidisciplinary approach for adequate treatment and follow-up.
Core Tip: Fibroadenoma (FA) is the most common tumor found in women. Although it can occur in any age group, is important to consider when to suspicious a malignant change of FA. The case we report is of great interest to the radiology community because ductal carcinoma in situ within a FA is a challenge to diagnosis. The aim of this case report is to present the current findings in imaging studies for an early diagnosis and to not delay the treatment.
- Citation: Olivares-Antúnez Y, Dávila-Zablah YJ, Vázquez-Ávila JR, Gómez-Macías GS, Mireles-Aguilar MT, Garza-Montemayor ML. Ductal carcinoma in situ within a fibroadenoma: A case report and review of literature. World J Radiol 2024; 16(3): 58-68
- URL: https://www.wjgnet.com/1949-8470/full/v16/i3/58.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i3.58
Ductal carcinoma in situ (DCIS) arising within a fibroadenoma (FA) is rarely encountered, although pure FA is the most common tumor found. Its incidence ranges from 0.02% to 0.125%, and it is incidentally found. We present a case of breast DCIS arising within a FA and discuss the imaging findings.
The 46-year-old female was diagnosed with a conglomerate of breast nodules which is stable for a period of 1 year. She came to our department for routine mammography and follow-up nodules by ultrasound (US).
She is asymptomatic.
Family history: Cervical cancer (her mother was diagnosed at age 81 and her sister was diagnosed at age 50). Gastric cancer (her grandmother diagnosed at age 80).
Personal history: The patient had a history of augmentation mammoplasty.
Right breast without palpable mass. Right and left axillary lymph nodes clinically negative.
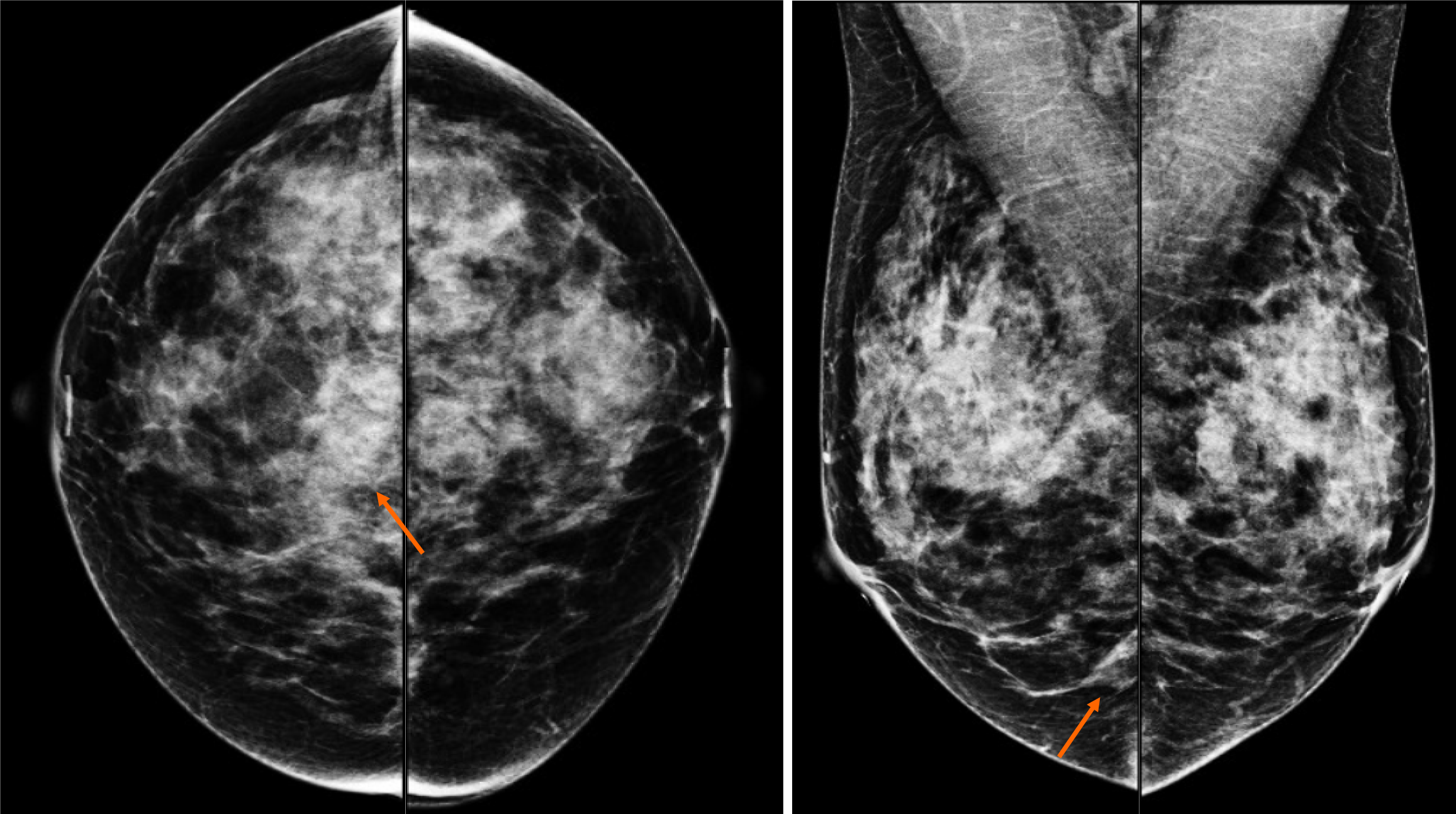
By mammography, the tissue breast is extremely dense (category “D” of the American College of Radiology, 2013). In the right breast, a conglomerate of nodules with obscured margins is seen at the posterior third of the union of lower quadrants. In both axillary regions, no lymph nodes are shown (Figure 1).
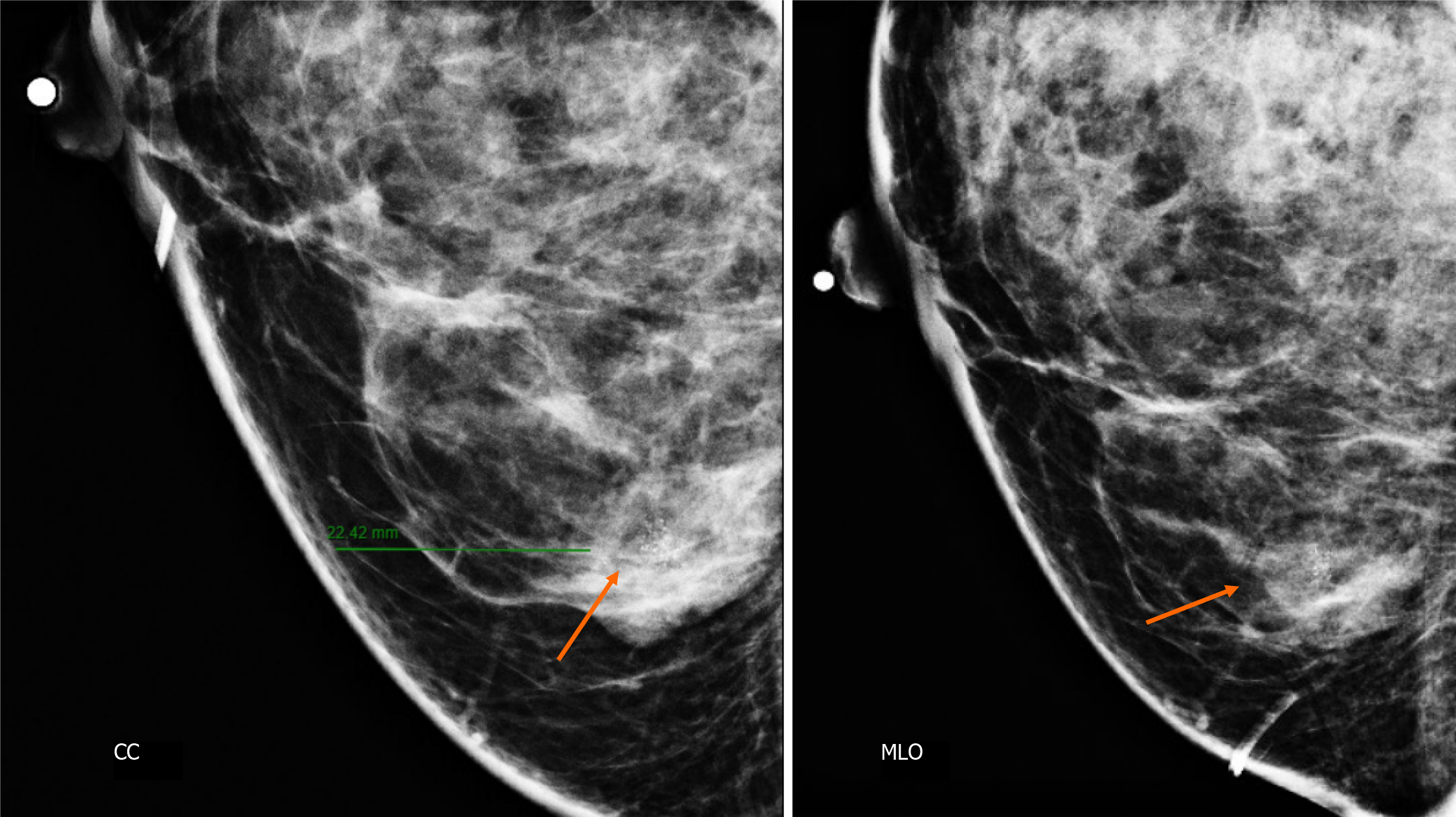
Craniocaudal and Mediolateral Oblique projections with magnification of right breast reveal two confluence nodules, which are isodense, oval with obscured margins and microcalcifications associated. The findings are localized 2 cm from skin adjacent to the mastoplasty surgical scar indicated by the linear tissue marker (Figure 2).
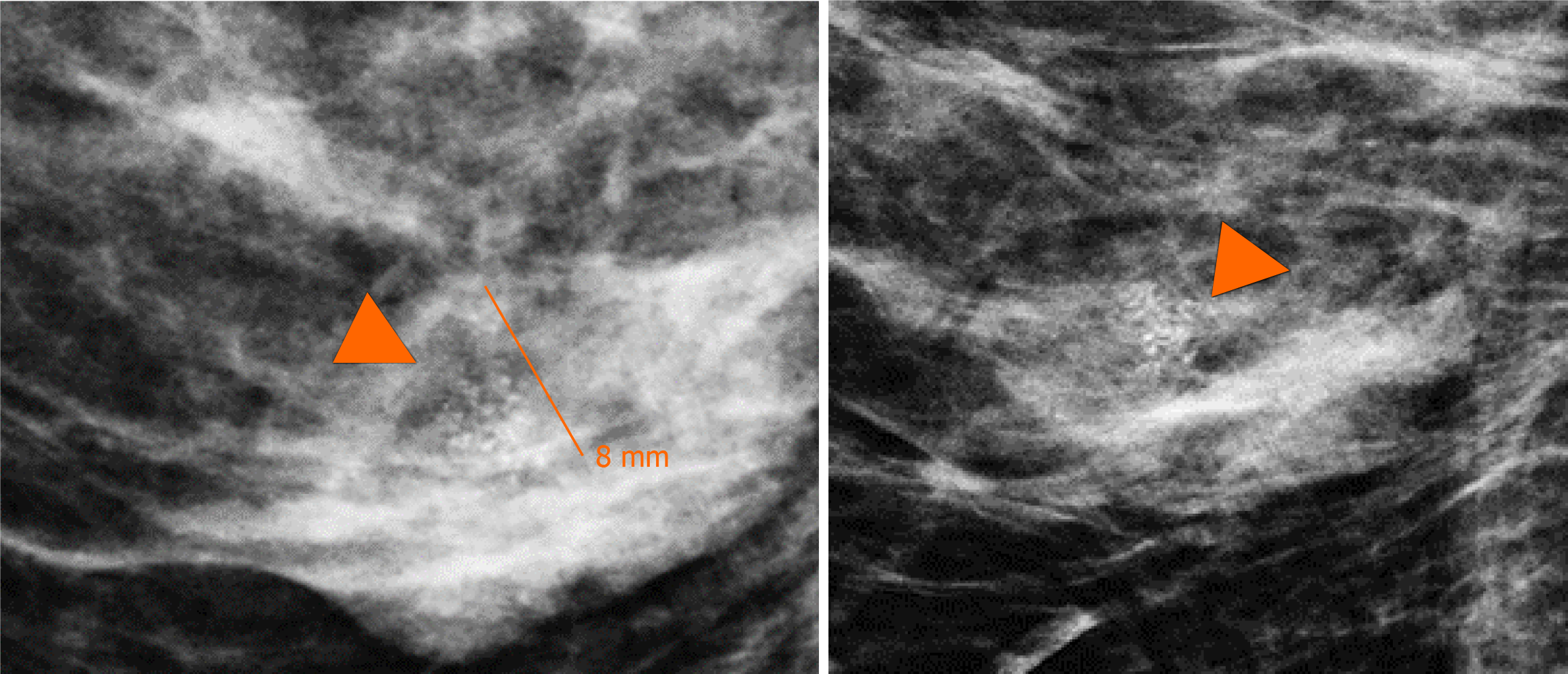
In addition, the magnification views in orthogonal projections reveal that one of the nodules is associated with fine and linear pleomorphic microcalcifications that extended in an area of 8 mm (Figure 3).
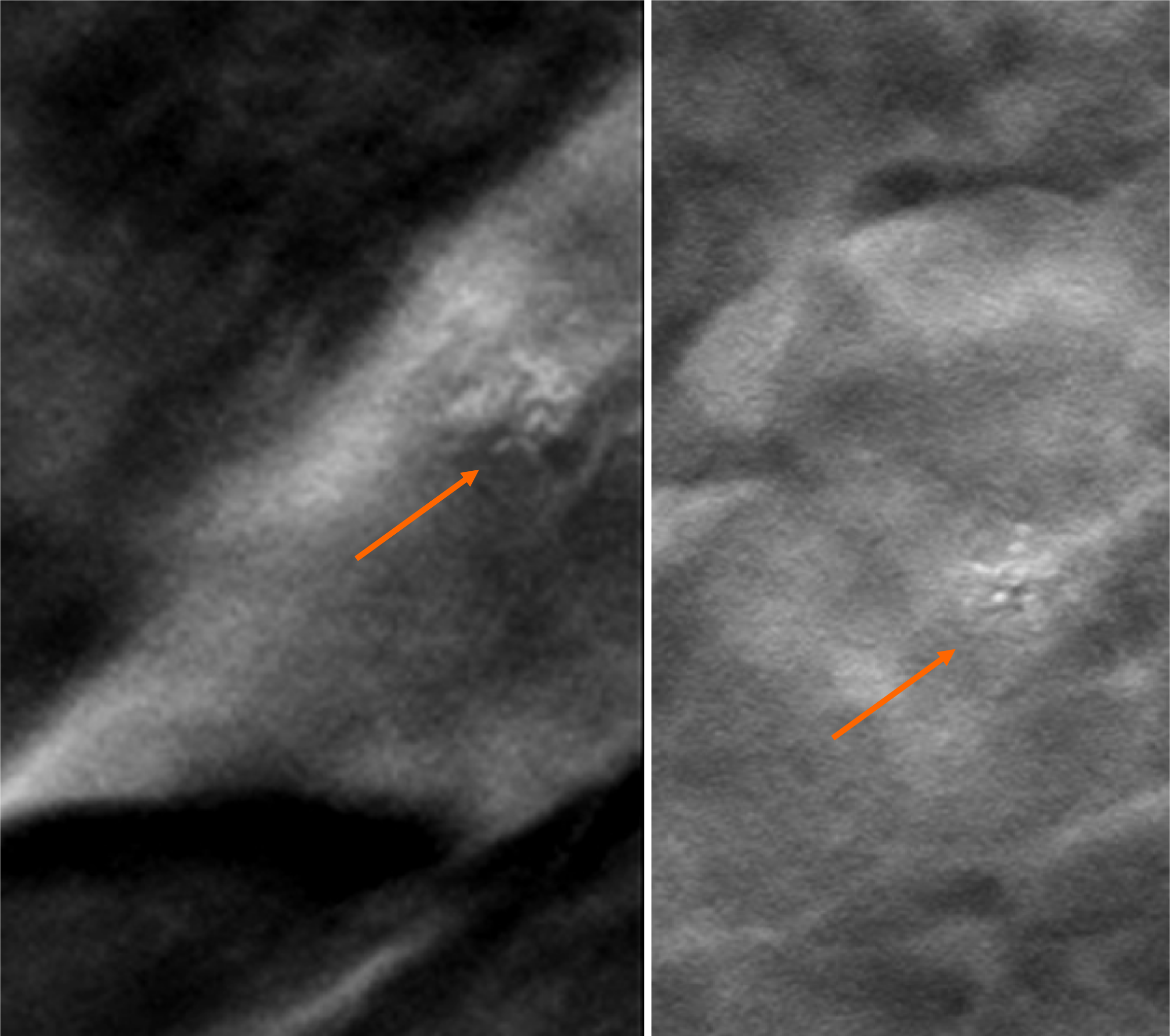
By digital breast tomosynthesis, we corroborate the morphology of microcalcifications (Figure 4).
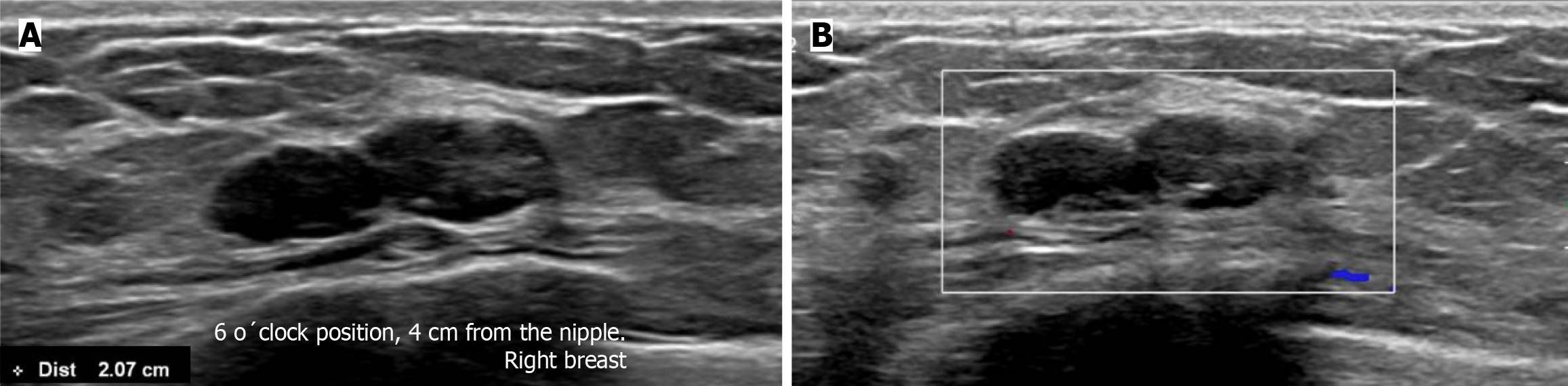
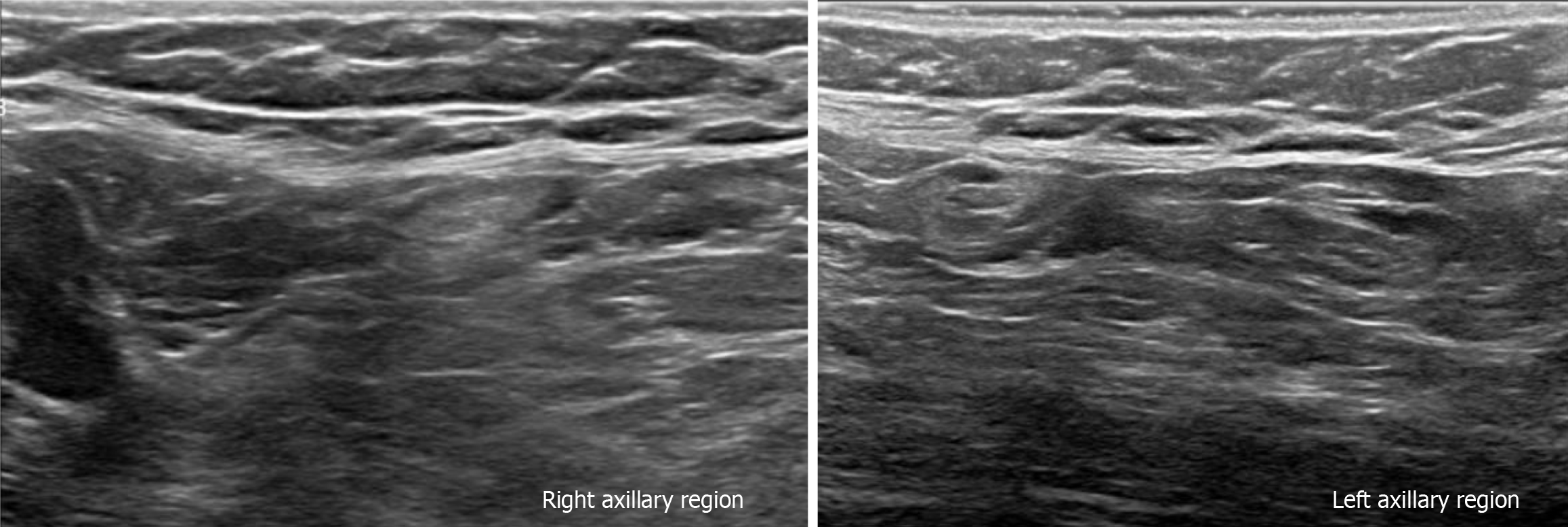
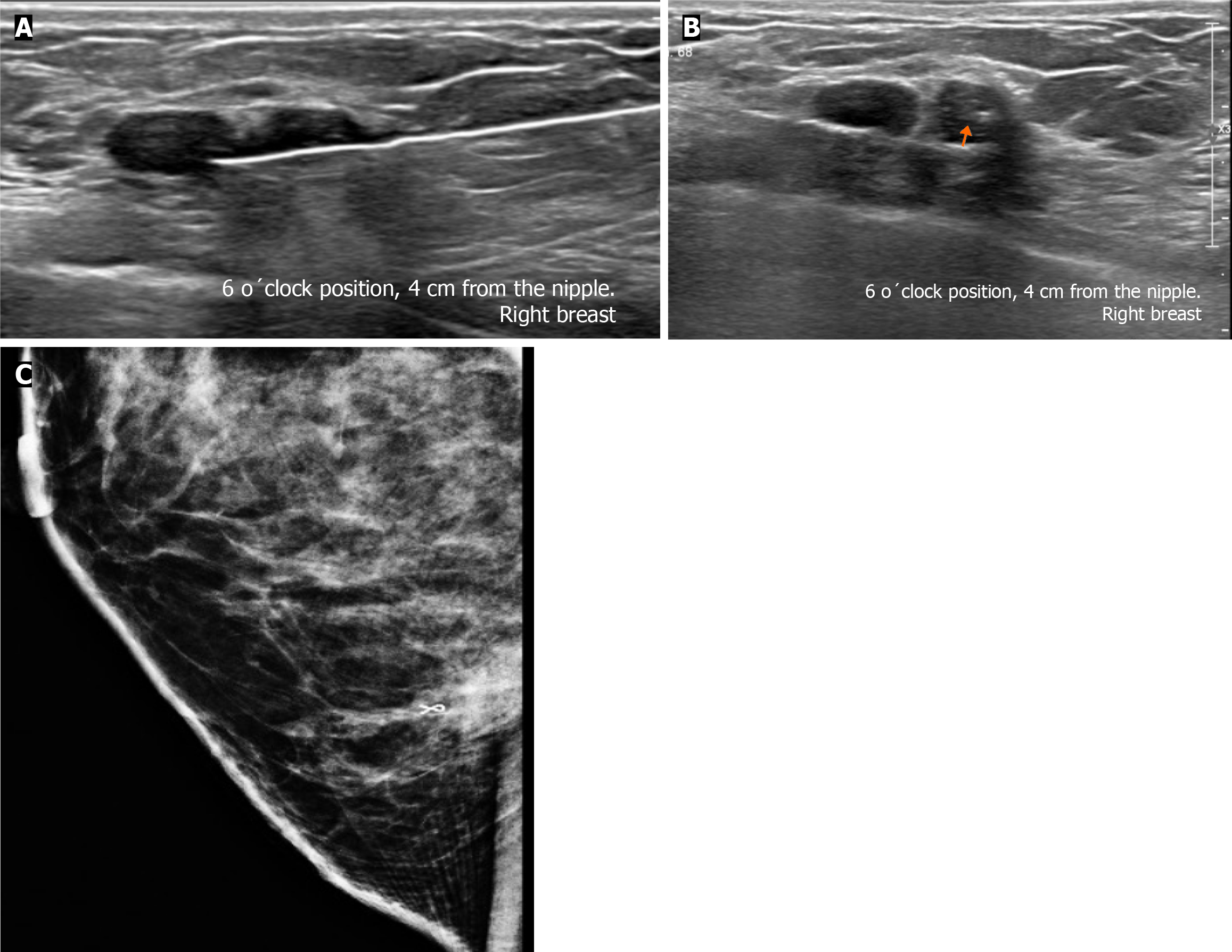
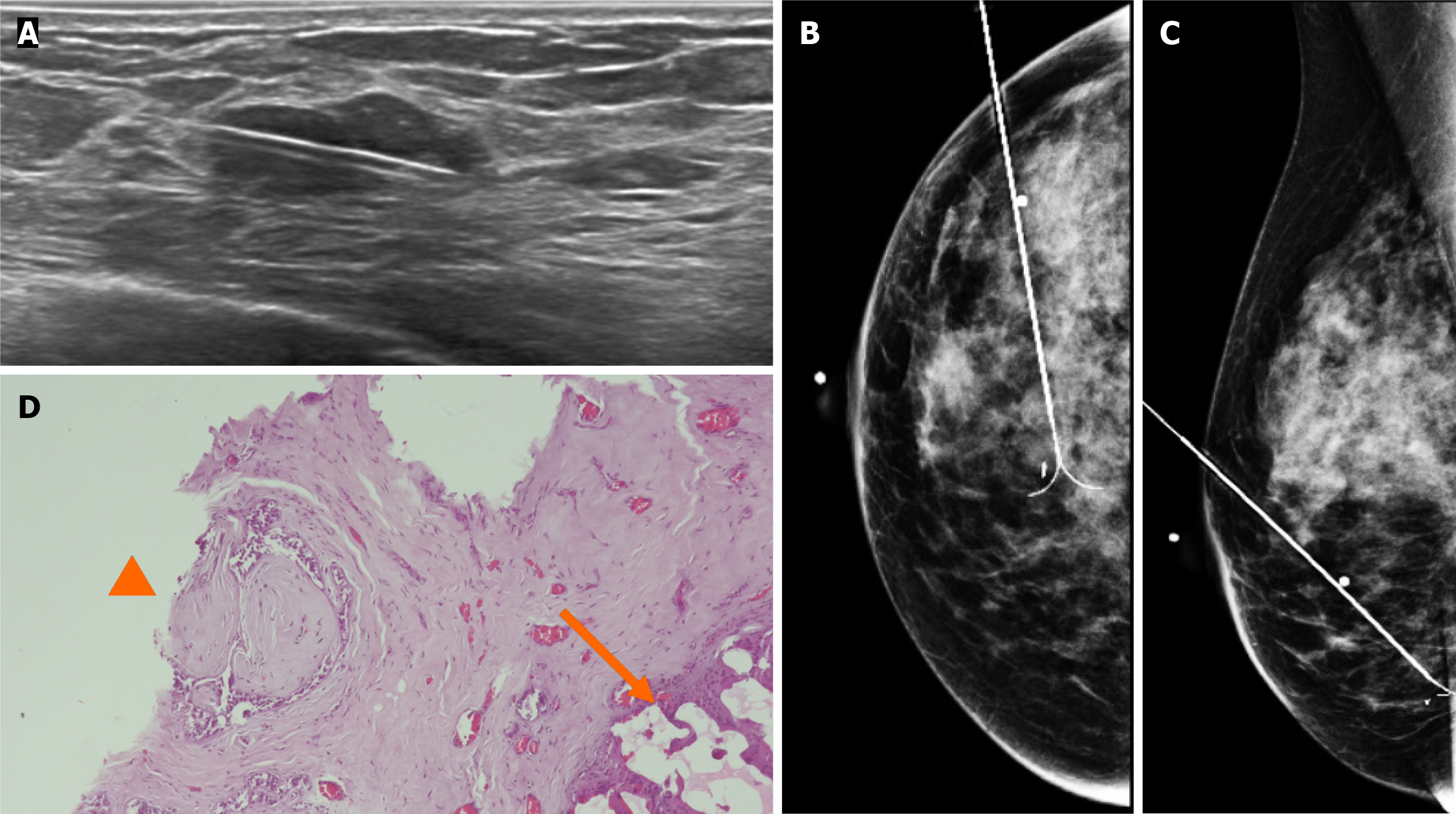
Subsequently, a bilateral breast US was performed. In the right breast is seen a conglomerate of two solid nodules that are hypoechoic, oval, circumscribed, avascular and non-palpable, located at the 6 o'clock position, 4 cm from the nipple in the right breast (Figure 5). One of the nodules shows microcalcifications seen in mammography. The bilateral axillary region demonstrates lymph nodes with morphology and fat hilum conservation (Figure 6). These findings correspond to the category of breast imaging-reporting and data system (BI-RADS) 4B.
An US-guided core biopsy was indicated. A percutaneous biopsy was performed of the conglomerate solid nodules, using a 12-gauze needle. Six cores were obtained and sent in formalin for pathology analysis. A tissue marker (or clip) is placed in one of the nodules which is associated with macrocalcifications. A projection of mammography is taken to confirm the position of the tissue marker (Figure 7).
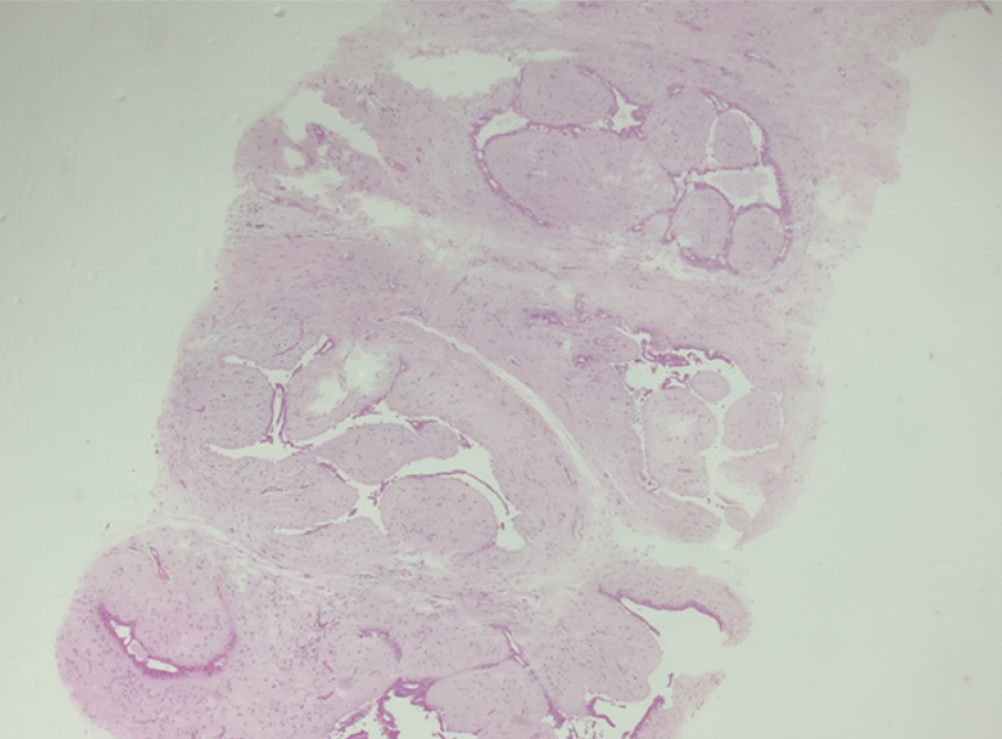
The histopathology report corresponds to 1.5 mm of high–nuclear grade DCIS with comedonecrosis and microcalcifications, which are within a fibroephitelial lesion that corresponds to a FA. Hematoxylin and eosin stain slides revealed pleomorphic neoplastic cells within a FA (Figure 8).
The patient underwent surgery, involving guidewire localization of the non-palpable conglomerate of nodules done under US (Figure 9). The treatment consists of conserving surgery and no sentinel lymph node biopsy was needed.
The pathological report corresponds to residual tumor of 0.5 mm foci which corresponds to a high–nuclear grade DCIS, comedo subtype, central necrosis and microcalcifications associated within a complex FA, that measures 2.5 cm.
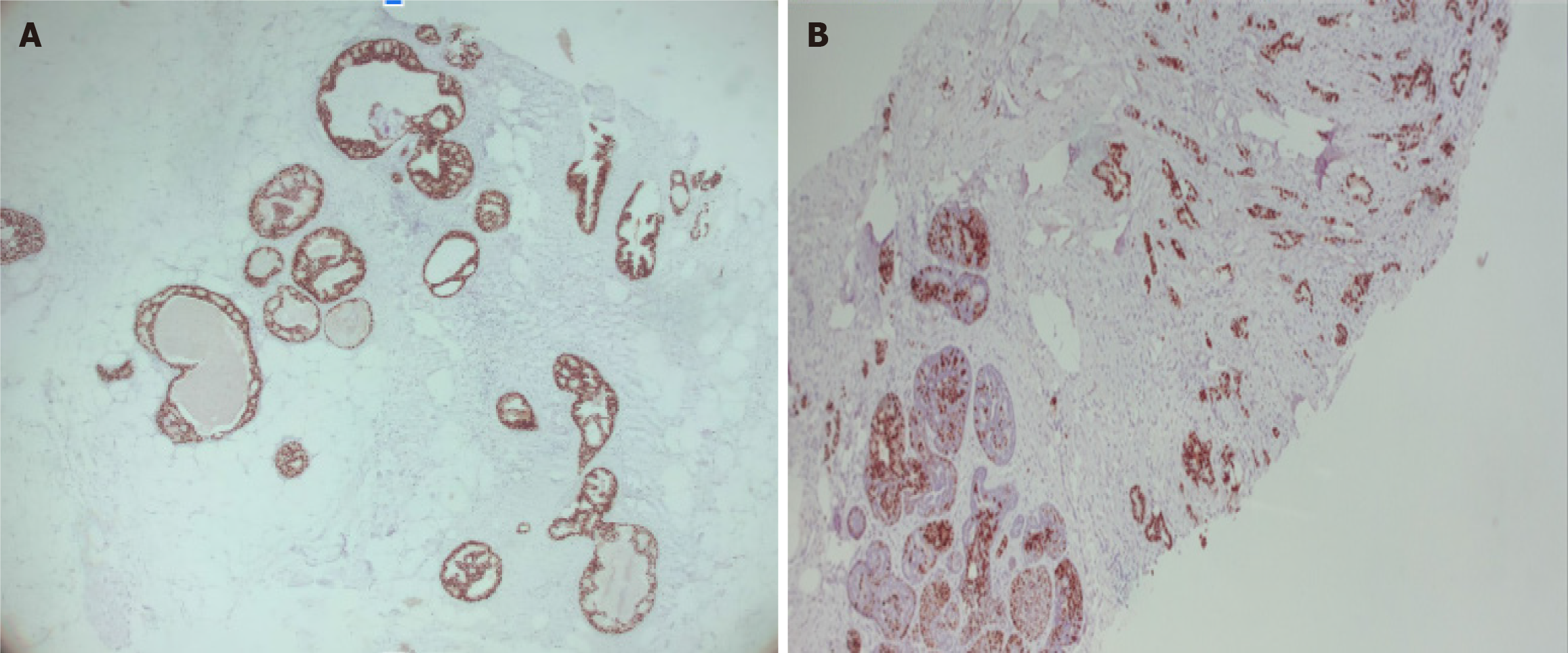
Stage 0, cTisN0M0 within a FA, with the following immunochemistry markers: Estrogen receptor (+) 90% and progesterone receptor (+) 70% (Figure 10).
Other histological findings: Columnar cell changes and hyperplasia with microcalcifications and 2 peripheral papillomas, one measures 0.7 mm.
The patient subsequently received radiotherapy and endocrine therapy (tamoxifen). According to the final diagnosis the patient has a good prognosis.
FA is the most common benign fibroepithelial breast tumor in young women and can occur at any age, being most common between 20 years and 40 years of age[1-13].
FA is a biphasic tumor composed of stromal (connective) and epithelial tissue that grows by estrogen and progesterone stimulation and is commonly found in pregnancy and lactation. During menopause, FAs suffer atrophy[3,4,6,9,13].
FAs are divided into simple and complex types. FAs with 1 or more of the following characteristics are considered complex: Epithelial calcifications, apocrine metaplasia, sclerosing adenosis and/or cysts larger than 3 mm[1,4-7,9,11,12,14].
The risk of breast carcinoma in complex FAs is 3.10 times greater than in patients with pure FAs and is associated with the percentage of epithelial proliferation[1,3,5,13].
DCIS within a breast FA is uncommon, with an incidence of 0.02% to 0.125%. Fewer than 130 cases have been reported in the literature[1].
Cancer may arise from the surrounding breast tissue, in the fissures of the FA, or the carcinoma may be completely confined within the FA. The age at presentation of these patients is 42.5 to 46.9 years-old, approximately 20 years later than the maximum age at presentation of patients with pure FA[1,6,10,12].
There are no specific imaging characteristics to distinguish between a pure FA and DCIS within a FA. In most cases, DCIS presents as an incidental finding and is indistinguishable from benign lesions on imaging (Table 1)[1].
| Diagnosis | Description of microcalcifications |
| Ductal carcinoma in situ within fibroadenoma | Fine or linear pleomorphic |
| Fat necrosis | Coarse calcifications and round or curvilinear calcifications in cyst wall |
| Initial degenerating fibroadenoma | Numerous, dense and peripherical located in a nodule and the coarse pathognomonic calcifications |
| Benign or malignant phyllodes tumor | Coarse, amorphous, and punctuate |
| Phyllodes tumor with carcinoma component | Benign-looking specks or suspicious coarse punctate |
| Triple-negative tumor | Clustered microcalcifications |
| Mucinous carcinoma | Round, coarse, amorphous and/or rarely pleomorphic |
| Papillary carcinoma | Pleomorphic, coarse, or stippled |
| Metaplastic carcinoma | Amorphous, fine pleomorphic, fine linear, and lastly coarse heterogeneous |
The suspicious finding of carcinoma in a FA in mammography is a group of fine or linear pleomorphic microcalcifications within a nodule of indistinct margins[1-4,6,7,9,12,14].
By US, if the shape or margin of the nodule is irregular and has acoustic shadowing, an echogenic halo or distortion of the architecture should be considered suspicious for malignancy[1-4,6,7].
By magnetic resonance imaging (MRI), a typical FA is observed as an oval mass, with circumscribed margins and persistent contrast enhancement. A carcinoma should be suspected when there is contrast enhancement with a rapid uptake curve and washout late phase (type 3)[1,3,6,7].
In adult patients, the American Society of Breast Surgeons recommends against routinely excising biopsy-proven FA that are < 2 cm. The American College of Radiology Appropriateness Criteria for palpable breast masses even states that short term imaging follow-up (such as every 6 months for 2 years) is a reasonable alternative to biopsy for solid masses with probably benign features suggesting FA[15].
A core biopsy should be performed on a nodule that presents rapid growth. The criteria for rapid growth are: (1) Volume growth rate ≥ 16% per month for patients younger than 50 years; (2) volume growth ≥ 13% per month for patients ≥ 50 years; and (3) mean change in dimension over a 6-month interval of > 20, especially in patients over 40 years of age to exclude the possibility of phyllodes tumor or malignancy[15].
The indications for excision include size > 30 mm, considering that pre-operative biopsy is also insufficient to distinguish phyllodes tumor from FA, and there is the possibility of underestimation. Other indications for surgical removal are for growing FA, a nodule with increased BI-RADS classification grade during the follow-up and core needle biopsy suggesting atypical hyperplasia or unusual pathologic features. Persistent discomfort and pain from a FA are a relative indication to consider surgical excision. Another indication for surgical removal is patient´s request or cosmetic concerns[6,16-19].
The differential diagnoses to consider for a nodule with suspicious microcalcifications are fat necrosis, initial degenerating FA, phyllodes tumor, triple-negative, mucinous, papillary, and metaplastic carcinoma (Table 1)[6,20].
Fat necrosis can be seen as a circumscribed soft-tissue mass with or without macro- or microcalcifications. At mammography, fat necrosis may be characterized by lipid cysts, microcalcifications, coarse calcifications near ar
The classic degenerating FA contains coarse pathognomonic calcifications. Occasionally, at the initial period of involution, the calcifications are small, numerous and may have a malignant appearance. Mammography helps to distinguish from ductal carcinoma. The microcalcifications in carcinomas are commonly small and asymmetrically located in a small area, whereas those in FAs tend to be denser, and more diffusely spaced or peripherical located in a nodule[20].
The phyllodes tumor is observed as a dense, round, or oval mass with circumscribed or indistinct margins. Liberman et al[22]. described 4 cases of benign and malignant phyllodes tumors containing coarse, amorphous, and punctuate microcalcifications. Also, the presence of benign-looking specks or suspicious coarse punctate clusters of microcalcifications is reported in less than a third (29%) of phyllodes tumors with a carcinoma component, which would significantly change the subsequent management plan. By US the phyllodes tumor is round or oval, heterogeneous with cystic areas and posterior acoustic enhancement. By MRI, it is a round, lobulated mass with circumscribed margins. When the tumor presents cystic areas, the mass is hypointense on T1-sequences and hyperintense on T2-sequences. Only 33% of these tumors show enhancement, including the internal septa[6,22,23].
Mucinous carcinoma occurs more frequently in older people, and the average age at presentation is 71 years. It re
The intraductal papillary neoplasms of the breast include papilloma, papilloma with atypical ductal hyperplasia (ADH) or DCIS, papillary DCIS (pDCIS), encapsulated papillary carcinoma (EPC), solid papillary carcinoma (SPC) and invasive papillary carcinoma (IPC). Papilloma, papilloma with ADH, DCIS, and pDCIS are associated with microcalcification. Papillary carcinomas with microcalcifications within the tumor are usually pleomorphic but may occasionally be coarse or stippled in appearance. US can reveal a hypoechoic solid mass or a complex cyst with septations or mural-based papilliform nodularity. The most common finding of EPC on mammography is a solitary oval or round mass with microlobulated or circumscribed margins and calcifications are not uncommon and are mainly amorphous or pleomorphic. In SPC concomitant microcalcification on mammography was rarely reported in the literature cases and 33.32% were accompanied by amorphous calcifications. Sonographically has been reported as multiple nodules accompanied by ductal ectasia, well-circumscribed, complex, cystic lesion, and homogeneous solid lesions. Ciurea et al[25]. reported IPCs as round or lobulated masses, often associated with mammographic calcification. Micropapillary DCIS is frequently associated with “snake skin-like” microcalcification[25-27].
In Krizmanich-Conniff et al[28] series found that 7% of triple-negative carcinoma appeared as clustered microcalcifications, whilst another 29% manifested as masses with associated microcalcifications. The rapid growth, with no precancerous stage, also explains the low incidence of microcalcifications. Microcalcifications inside a mass or isolated segmental type calcifications were more often associated with a DCIS and a human epidermal growth factor receptor 2+ status. On US and MRI images, commonly theses tumors appear as a round or oval mass with irregular, spiculated, or circumscribed margins with rapid early enhancement and a late washout curve[6,28,29].
Metaplastic breast cancer, on mammography, are commonly large masses of 4.2 cm, with high density and smooth, well-defined and spiculated margins. Microcalcifications are very rare within the mass; when this happens, they are more likely to be amorphous, fine pleomorphic, fine linear, and lastly coarse heterogeneous[30,31].
It is important to understand that suspicious microcalcifications within masses can indicate the presence of ma
The treatment of choice is conservative surgery, if 2 or fewer suspicious lymph nodes are found on imaging, or 2 or fewer positive lymph nodes are confirmed by needle biopsy, then is recommended sentinel lymph node mapping. Adjuvant therapy includes radiotherapy and endocrine therapy. The majority have good prognosis, with no difference between young and older women. Ten percent of patients present with recurrence or metastasis[1-3,6,9].
FAs are common in imaging studies, but the presence of a DCIS within the nodule is rare and has nonspecific findings on imaging. Faced with a circumscribed nodule within microcalcifications is a diagnostic challenge and by imaging studies can simulate benign and malignant pathologies. The radiologist has to consider in the differential diagnosis DCIS within a FA or other round tumors associated with microcalcifications, and the core biopsy should be performed. We re
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Tangsuwanaruk T, Thailand; Yap RV, Philippines S-Editor: Luo ML L-Editor: A P-Editor: Zhao S
| 1. | Ni XH, An R, Shi QW, Wang CL. Low-Grade Ductal Carcinoma in situ Within a Fibroadenoma of the Breast: A Rare Case Report and Review of the Literature. Onco Targets Ther. 2023;16:399-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 2. | Wu J, Sun KW, Mo QP, Yang ZR, Chen Y, Zhong MC. Preoperational diagnosis and management of breast ductal carcinoma in situ arising within fibroadenoma: Two case reports. World J Clin Cases. 2022;10:3496-3504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 1] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 3. | Hammood ZD, Mohammed SH, Abdulla BA, Omar SS, Naqar S, Salih AM, Kakamad FH. Ductal carcinoma in situ arising from fibroadenoma; a rare case with review of literature. Ann Med Surg (Lond). 2022;75:103449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 4. | Brunetti N, Valente I, Pitto F, Calabrese M. Triple-negative Breast Cancer Arising in a Fibroadenoma in BRCA 1 Mutated Patient. J Med Ultrasound. 2023;31:63-65. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 5. | Shojaku H, Hori R, Yoshida T, Matsui K, Shimada K, Takayanagi N, Noguchi K. Low-grade ductal carcinoma in situ (DCIS) arising in a fibroadenoma of the breast during 5 years follow-up: A case report. Medicine (Baltimore). 2021;100:e24023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Feliciano YZ, Freire R, Net J, Yepes M. Ductal and lobular carcinoma in situ arising within an enlarging biopsy proven fibroadenoma. BMJ Case Rep. 2021;14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Kato F, Omatsu T, Matsumura W, Takahashi M, Hosoda M, Takahashi H, Kubota K, Oyama-Manabe N, Terae S, Shirato H. Dynamic MR findings of ductal carcinoma in situ within a fibroadenoma. Magn Reson Med Sci. 2011;10:129-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Fukuda M, Nagao K, Nishimura R, Matsuda M, Baba K, Ueno Y, Morinaga H, Omachi H, Hamada T. Carcinoma arising in fibroadenoma of the breast--a case report and review of the literature. Jpn J Surg. 1989;19:593-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | El-Essawy M, AL Haidary A, Khan AL. Ductal carcinoma in situ (DCIS) in breast fibroadenoma. Egypt J Radiol Nucl Med 51. 2020;73. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Ren Y, Li P, Yang Y, Huang Y, Zhan D, Wen B. Imaging findings of ductal carcinoma in situ arising within fibroadenoma. Breast J. 2020;26:1037-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Fujimoto A, Matsuura K, Kawasaki T, Ichinose Y, Nukui A, Hiratsuka M, Asano A, Shimada H, Osaki A, Saeki T. Early HER2-positive breast cancer arising from a fibroadenoma: a case report. Oxf Med Case Reports. 2021;2021:omab083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 12. | Borecky N, Rickard M. Preoperative diagnosis of carcinoma within fibroadenoma on screening mammograms. J Med Imaging Radiat Oncol. 2008;52:64-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Dupont WD, Page DL, Parl FF, Vnencak-Jones CL, Plummer WD Jr, Rados MS, Schuyler PA. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med. 1994;331:10-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 246] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Baker KS, Monsees BS, Diaz NM, Destouet JM, McDivitt RW. Carcinoma within fibroadenomas: mammographic features. Radiology. 1990;176:371-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Harvey JA, Mahoney MC, Newell MS, Bailey L, Barke LD, D'Orsi C, Hayes MK, Jokich PM, Lee SJ, Lehman CD, Mainiero MB, Mankoff DA, Patel SB, Reynolds HE, Sutherland ML, Haffty BG. ACR Appropriateness Criteria Palpable Breast Masses. J Am Coll Radiol. 2016;13:e31-e42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Sklair-Levy M, Sella T, Alweiss T, Craciun I, Libson E, Mally B. Incidence and management of complex fibroadenomas. AJR Am J Roentgenol. 2008;190:214-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Peng Y, Xie F, Zhao Y, Wang S; Chinese Society of Breast Surgery. Clinical practice guideline for breast fibroadenoma: Chinese Society of Breast Surgery (CSBrS) practice guideline 2021. Chin Med J (Engl). 2021;134:1014-1016. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Kopkash K, Yao K. The surgeon’s guide to fibroadenomas. Ann Breast Surg. 2020;4:25-25. [DOI] [Cited in This Article: ] |
| 19. | Gillette DP, He J, Lee AE, Chao C. Indications for the surgical excision of fibroadenomas: systematic review. AME Surg J. 2023;3:3. [DOI] [Cited in This Article: ] |
| 20. | Ranveer SA, Biren AS, Dongping S. Mucinous Carcinoma of the breast: Mammography and Ultrasound Appearance with Histopathology Correlation. JOJ Case Stud. 2019;9:555774. [DOI] [Cited in This Article: ] |
| 21. | Kerridge WD, Kryvenko ON, Thompson A, Shah BA. Fat Necrosis of the Breast: A Pictorial Review of the Mammographic, Ultrasound, CT, and MRI Findings with Histopathologic Correlation. Radiol Res Pract. 2015;2015:613139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Liberman L, Bonaccio E, Hamele-Bena D, Abramson AF, Cohen MA, Dershaw DD. Benign and malignant phyllodes tumors: mammographic and sonographic findings. Radiology. 1996;198:121-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 124] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Yuen WN, Li JJX, Chan MY, Tse GM. High-grade ductal carcinoma in-situ detected by microcalcification within borderline phyllodes tumor: Report of a case and literature review. Hum Pathol Repo. 2023;31:300697. [DOI] [Cited in This Article: ] |
| 24. | Shin GW, Park HY, Park YM. Mucinous Breast Carcinoma Presenting as a Coarse and Densely Calcified Mass on Mammography: A Case Report. Taehan Yongsang Uihakhoe Chi. 2020;81:1266-1271. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 25. | Ciurea A, Calin A, Ciortea C, Dudea SM. Ultrasound in the diagnosis of papillary breast lesions. Med Ultrason. 2015;17:392-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Kurtoğlu Özçağlayan Tİ, Öznur M. Digital Mammography, Ultrasound and Magnetic Resonance Imaging Characteristics in Differential Diagnosis of Papillary Carcinoma Subtypes of the Breast and Diagnostic Challenges. Eur J Breast Health. 2022;18:172-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Kulka J, Madaras L, Floris G, Lax SF. Papillary lesions of the breast. Virchows Arch. 2022;480:65-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Krizmanich-Conniff KM, Paramagul C, Patterson SK, Helvie MA, Roubidoux MA, Myles JD, Jiang K, Sabel M. Triple receptor-negative breast cancer: imaging and clinical characteristics. AJR Am J Roentgenol. 2012;199:458-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Boisserie-Lacroix M, Mac Grogan G, Debled M, Ferron S, Asad-Syed M, Brouste V, Mathoulin-Pelissier S, Hurtevent-Labrot G. Radiological features of triple-negative breast cancers (73 cases). Diagn Interv Imaging. 2012;93:183-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Corso G, Criscitiello C, Nicosia L, Pesapane F, Vicini E, Magnoni F, Sibilio A, Zanzottera C, De Scalzi AM, Mannucci S, Marabelli M, Calvello M, Feroce I, Zagami P, Porta FM, Toesca A, Tarantino P, Nicolò E, Mazzarol G, La Vecchia C, Bonanni B, Leonardi MC, Veronesi P, Fusco N. Metaplastic breast cancer: an all-round multidisciplinary consensus. Eur J Cancer Prev. 2023;32:348-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 31. | Asare B, White MJ, Rossi J. Metaplastic carcinoma with osteosarcomatous differentiation in the breast: Case report. Radiol Case Rep. 2023;18:4272-4280. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |