Copyright
©The Author(s) 2016.
World J Cardiol. Dec 26, 2016; 8(12): 695-702
Published online Dec 26, 2016. doi: 10.4330/wjc.v8.i12.695
Published online Dec 26, 2016. doi: 10.4330/wjc.v8.i12.695
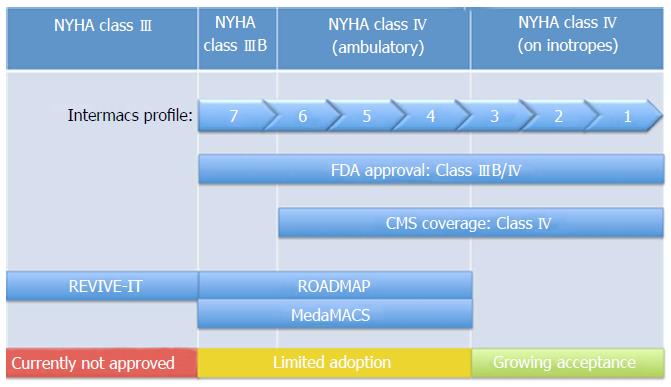
Figure 2 New York Heart Association classes considered for left ventricular assist devices implantation.
Currently, FDA approval for LVAD implantation exists for NYHA Class IIIB and IV, which encompasses all of the INERMACS profile levels. ROADMAP is evaluating LVAD implantation in patients of NYHA class III and class IV (ambulatory), which has limited adoption in most clinical practices. MedaMACS looked at the same patient population as ROADMAP however focused on those patients without LVADs. REVIVE-IT was evaluating implantation in patients in NYHA class III, which is not currently FDA approved. LVAD: Left ventricular assist devices; FDA: Food and Drug Administration; MedaMACS: Medical Arm of the Interagency Registry for Mechanically Assisted Circulatory Support; NYHA: New York Heart Association.
- Citation: Cerier E, Lampert BC, Kilic A, McDavid A, Deo SV, Kilic A. To ventricular assist devices or not: When is implantation of a ventricular assist device appropriate in advanced ambulatory heart failure? World J Cardiol 2016; 8(12): 695-702
- URL: https://www.wjgnet.com/1949-8462/full/v8/i12/695.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i12.695