Copyright
©The Author(s) 2015.
World J Cardiol. May 26, 2015; 7(5): 277-286
Published online May 26, 2015. doi: 10.4330/wjc.v7.i5.277
Published online May 26, 2015. doi: 10.4330/wjc.v7.i5.277
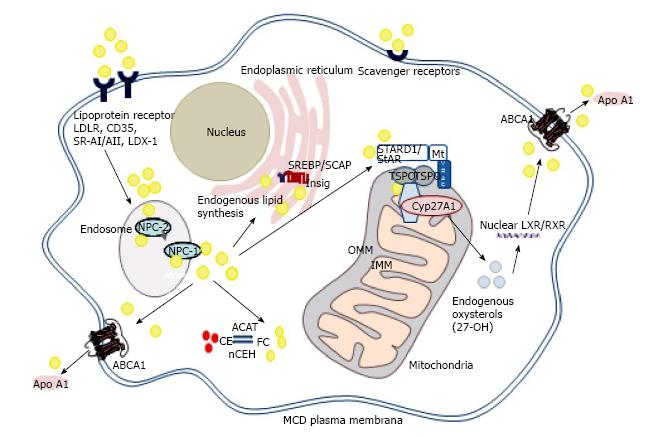
Figure 1 The role of mitochondrial cholesterol trafficking in regulation of macrophage sterol metabolism.
Increased expression of steroidogenic acute regulatory protein (StAR, STARD1) or 18 kDa translocator protein (TSPO) drive cholesterol trafficking to mitochondrial sterol 27-hydroxylase (CYP27A1), enhancing endogenous production of oxysterols (24-, 25- and 27-hydroxycholesterol), in turn activating liver X receptors (LXR) and enhancing cholesterol efflux to apolipoprotein A-I (Apo AI) via ATP binding cassette transporter A1 (ABCA1). One current model for cholesterol transfer from the outer (OMM) to inner (IMM) mitochondrial membrane, derived from studies in steroidogenic cells, involves a complex of proteins, including StAR, TSPO, voltage-dependent anion channel (VDAC), regulatory subunits of protein kinase A (PKA-R1α), acyl CoA binding domains-1 and -3, ATPase family AAA domain-containing protein 3A (ASTAD3A) and optic atrophy type 1 proteins. Exogenous cholesterol delivered to the endocytic pathway via lipoprotein or scavenger receptors is transported either to the plasma membrane, enhancing cholesterol efflux via ABCA1, to lipid poor acceptors such as Apo AI or Apo E, or delivered to the endoplasmic reticulum (ER), retaining the Sterol Regulatory Element Binding Protein (SREBP)/SREBP-cleavage activating protein (SCAP) complex, in turn reducing cholesterol biosynthesis. Oxysterols enhance this process by binding to Insig-1/2 (insulin-induced gene-1 or -2). Excess cholesterol is esterified via Acyl CoA: Cholesterol Acyltransferase-1 (ACAT-1), and stored in lipid droplets within the cytoplasm as “foamy” droplets. nCEH: Neutral cholesteryl ester hydrolase; FC: Free cholesterol; CE: Cholesteryl ester: NPC-1/NPC-2: Niemann-Pick C1/C2 protein; StAR: Steroidogenic acute regulatory protein: RXR: Retinoic acid receptor.
- Citation: Graham A, Allen AM. Mitochondrial function and regulation of macrophage sterol metabolism and inflammatory responses. World J Cardiol 2015; 7(5): 277-286
- URL: https://www.wjgnet.com/1949-8462/full/v7/i5/277.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i5.277