Copyright
©2012 Baishideng Publishing Group Co.
World J Cardiol. Apr 26, 2012; 4(4): 90-102
Published online Apr 26, 2012. doi: 10.4330/wjc.v4.i4.90
Published online Apr 26, 2012. doi: 10.4330/wjc.v4.i4.90
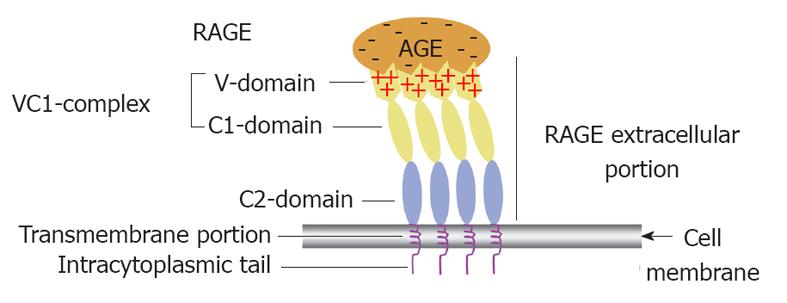
Figure 3 Receptor for advanced glycation end product (i.
e., colon) receptor structure and its functional implication in binding different advanced glycation end products. A diagrammatic illustration of the structure of the receptor for advanced glycation end product (RAGE) showing that it is composed of an extracelluar portion, a transmembrane portion and an intracytoplasmic tail. The extracelluar portion comprises three domain V, C1 and C2. The first two are believed to work together as a single functional complex (VC1) whereas the C2 domain remains attached to the VC1 complex but works independently from it. The diagram also illustrates how multiple RAGE receptors polymerise within the cell membrane to facilitate high affinity binding of the positively charged V domain with the negatively charged advanced glycation end products (AGEs) independent of their chemical structure. That is why RAGE is considered one of the pattern recognition receptors.
- Citation: Hegab Z, Gibbons S, Neyses L, Mamas MA. Role of advanced glycation end products in cardiovascular disease. World J Cardiol 2012; 4(4): 90-102
- URL: https://www.wjgnet.com/1949-8462/full/v4/i4/90.htm
- DOI: https://dx.doi.org/10.4330/wjc.v4.i4.90