Copyright
©The Author(s) 2025.
World J Cardiol. Feb 26, 2025; 17(2): 103733
Published online Feb 26, 2025. doi: 10.4330/wjc.v17.i2.103733
Published online Feb 26, 2025. doi: 10.4330/wjc.v17.i2.103733
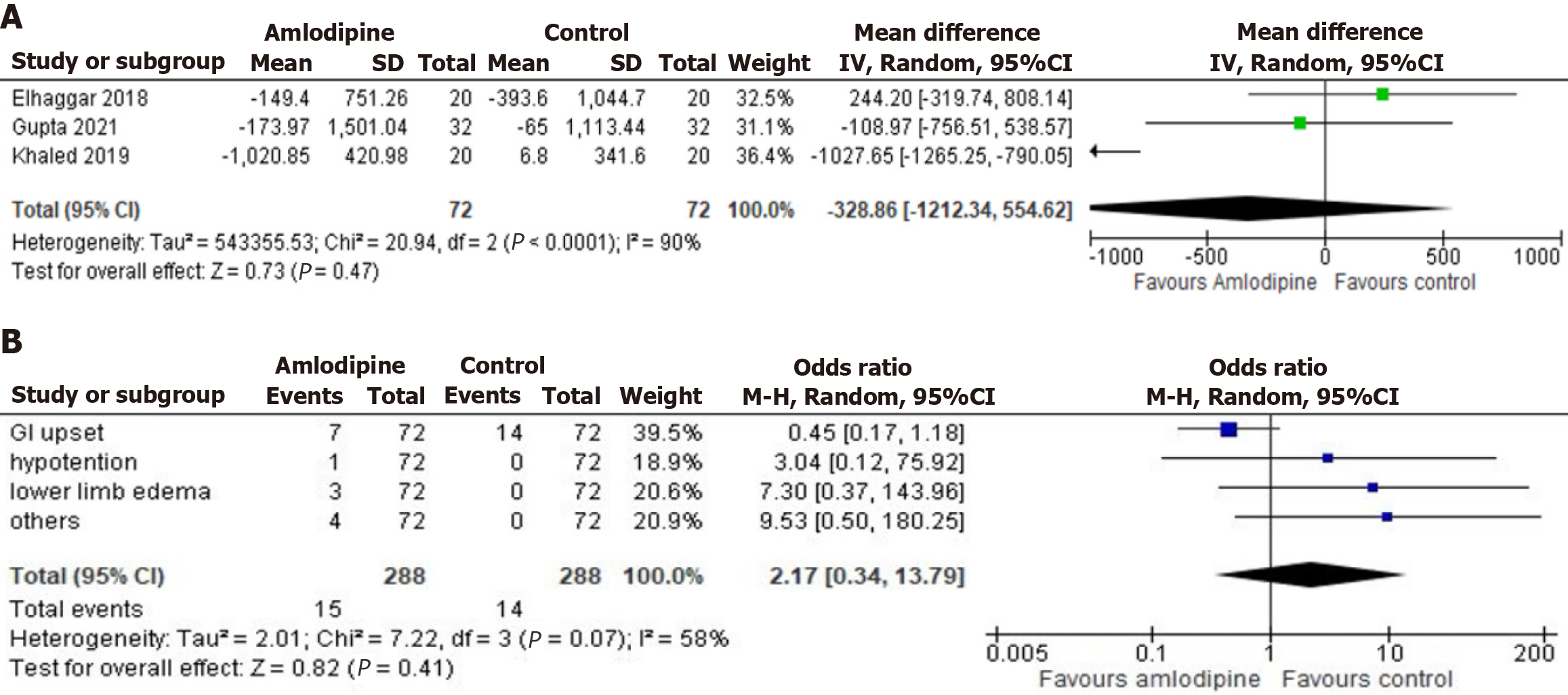
Figure 4 The forest plot of serum ferritin levels and reported adverse events.
A: The forest plot illustrates the non-significant changes in serum ferritin levels observed in the amlodipine group compared to the control group from baseline to follow-up. The analysis indicated that amlodipine resulted in a non-significant reduction in serum ferritin, with a mean decrease of 328.86 and a 95% confidence interval ranging from -1212.34 to 554.62. The heterogeneity among the studies, evaluated using the I2 statistic, was found to be 90%, which suggests a high level of variability in the outcomes across the included studies; B: The forest plot illustrates the odds ratio for reported adverse events, including gastrointestinal upset, hypotension, and lower limb edema, in the amlodipine group compared to the control group. The analysis demonstrated a statistically non-significant increased risk of adverse events in the amlodipine group, with an odds ratio of 2.17 and a 95% confidence interval ranging from 0.34 to 13.79. The heterogeneity among the studies, evaluated using the I2 statistic, was found to be 58%, indicating moderate variability in outcomes across the included studies. CI: Confidence interval; GI: Gastrointestinal.
- Citation: Safwan M, Bourgleh MS, Alsudays A, Haider KH. Combinatorial approach to treat iron overload cardiomyopathy in pediatric patients with thalassemia-major: A systematic review and meta-analysis. World J Cardiol 2025; 17(2): 103733
- URL: https://www.wjgnet.com/1949-8462/full/v17/i2/103733.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i2.103733