Published online Apr 26, 2024. doi: 10.4330/wjc.v16.i4.181
Peer-review started: December 13, 2023
First decision: January 15, 2024
Revised: January 26, 2024
Accepted: March 4, 2024
Article in press: March 4, 2024
Published online: April 26, 2024
Hypoxia-inducible factor 1 (HIF1) has a crucial function in the regulation of oxygen levels in mammalian cells, especially under hypoxic conditions. Its importance in cardiovascular diseases, particularly in cardiac ischemia, is because of its ability to alleviate cardiac dysfunction. The oxygen-responsive subunit, HIF1α, plays a crucial role in this process, as it has been shown to have cardioprotective effects in myocardial infarction through regulating the expression of genes affecting cellular survival, angiogenesis, and metabolism. Furthermore, HIF1α expression induced reperfusion in the ischemic skeletal muscle, and hypoxic skin wounds in diabetic animal models showed reduced HIF1α expression. Increased expression of HIF1α has been shown to reduce apoptosis and oxidative stress in cardiomyocytes during acute myocardial infarction. Genetic variations in HIF1α have also been found to correlate with altered responses to ischemic cardio
Core Tip: Hypoxia-inducible factor 1 (HIF1), a versatile transcription factor, is crucial for the maintenance of oxygen homeostasis. Genetic variations in HIF1α may influence tissue response to hypoxia and affect clinical manifestations of coronary atherosclerosis. Research has confirmed that sufficient HIF1α expression leads to reperfusion in the ischemic skeletal muscle, whereas decreased expression is associated with hypoxic skin wounds in diabetic animal models. In addition, the HIF1α response can be influenced by circadian proteins. Interpretation of circadian and hypoxia signaling pathways may enable therapeutic interventions in diseases associated with oxygen deprivation, including myocardial infarction.
- Citation: Škrlec I, Kolomeichuk SN. Hypoxia-inducible factor-1α in myocardial infarction. World J Cardiol 2024; 16(4): 181-185
- URL: https://www.wjgnet.com/1949-8462/full/v16/i4/181.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i4.181
Hypoxia-inducible factor 1 (HIF1) is a central regulator of oxygen homeostasis in mammalian cells and is activated under hypoxic conditions[1]. Hypoxia is a hallmark of many physiological and pathological conditions, and a stable HIF1α protein is essential for the adaptation and survival of cells in an oxygen-deprived environment – hypoxia[2]. In addition, HIF1 contributes to several hypoxia-related diseases, including cardiovascular diseases[1]. Oxidative metabolism is essential for the maintenance of cardiac contractility as it produces a large amount of ATP. Therefore, the heart is extremely sensitive to hypoxia, and myocardial ischemia is the leading cause of death in developed countries[3]. Oxygen-sensitive signaling pathways, such as HIF1α, are important for adapting to changes in oxygen availability during myocardial ischemia. HIF1α protein levels are regulated post-transcriptionally and are inversely proportional to oxygen levels[4]. HIF1α is involved in vascular responses to hypoxia, such as ischemia-induced angiogenesis and lipid metabolism, glucose catabolism, and redox homeostasis. The genetic variability of HIF1α is associated with cardiovascular diseases, such as coronary heart disease, ischemic heart disease, preeclampsia, and acute myocardial infarction[2].
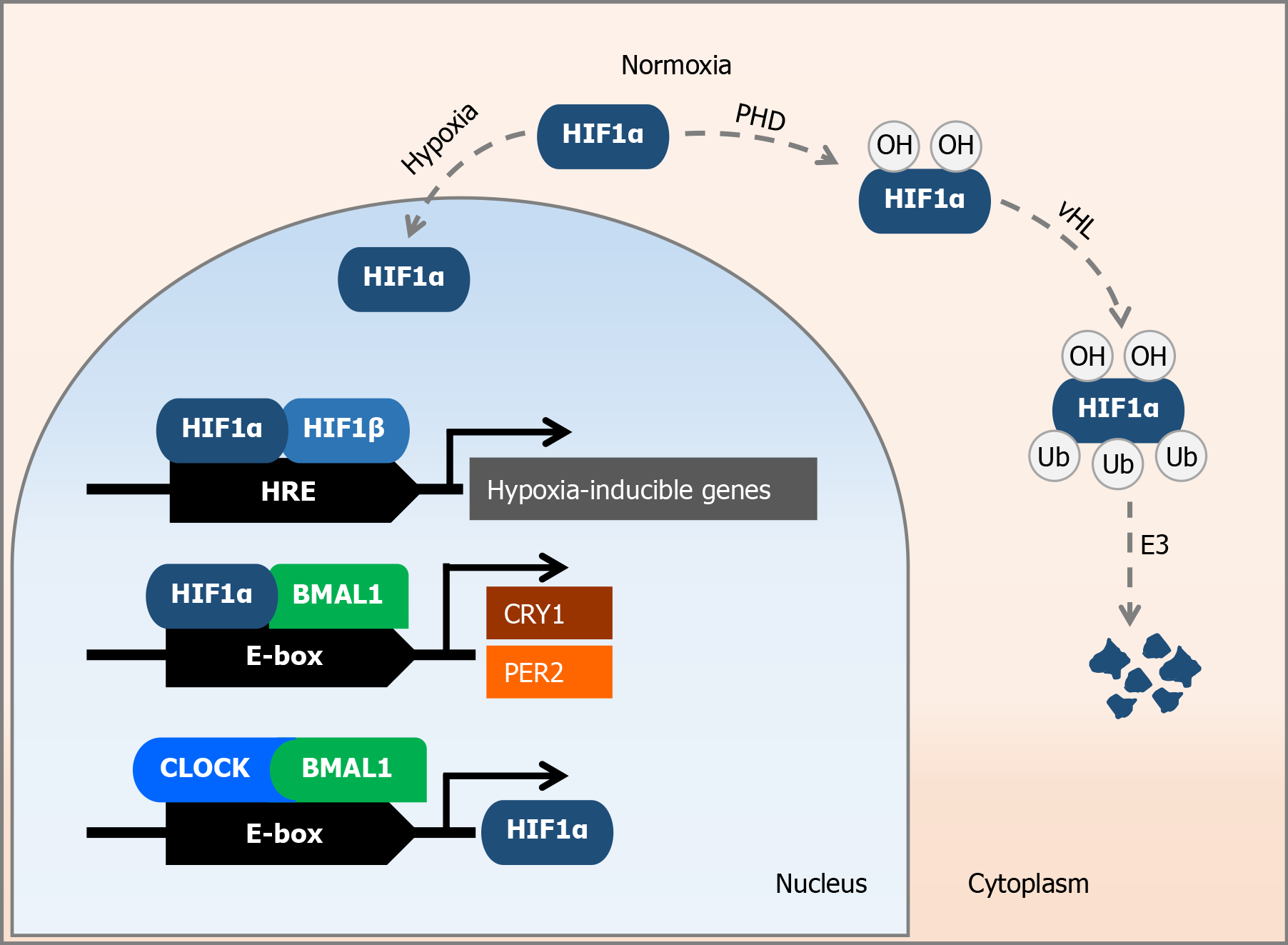
HIF1 is a transcription factor that consists of two subunits, α and β. The HIF1α subunit is oxygen-sensitive, whereas HIF1β is constitutively expressed[3]. The gene sequence encoding the HIF1α subunit is located on the long arm of chromosome 14 (14q23.2) and plays an important role in regulating cellular processes to maintain oxygen homeostasis[5]. In mammals, there are three different variants of the HIFα protein, with HIF1α being ubiquitously expressed in all cells, whereas the expression of HIF2α and HIF3α varies according to cell type and tissue[2]. Under conditions of oxygen deprivation - hypoxia - the expression of most genes is repressed at the transcriptional level. In contrast, the expression of a specific group of genes, the so-called hypoxia-inducible genes, is increased under hypoxic conditions[6]. These genes include erythropoietin, vascular endothelial growth factor, and genes involved in cell metabolism and inflammation[7]. Under normoxia, HIF1α is subject to oxygen-dependent hydroxylation. It is degraded by prolyl hydroxylase, an E3 ubiquitin ligase, and by the von Hippel-Lindau degradation pathway in the ubiquitin-proteasome system[1,4]. Under hypoxic conditions, HIF1α is prevented from degradation, accumulates, and migrates to the nucleus[6]. In the nucleus, the α-subunit of HIF1 forms a heterodimer with the β-subunit, resulting in a transcription factor that promotes cell survival, angiogenesis, and glycolysis[8]. HIF1α binds to hypoxia-responsive elements in the nucleus and activates the transcription of hypoxia-inducible genes[6] (Figure 1). It also stimulates gene transcription by binding to a specific DNA sequence 5'-RCGTG-3' (where R can be an A or G) within the hypoxia-responsive elements[9]. HIF1α stimulates the transcription of genes responsible for the production of enzymes, transporters, and mitochondrial proteins. These genes contribute to the reduction in oxygen consumption and control the transition of cells from oxidative to glycolytic metabolism[9]. When oxygen levels are reduced, the degradation of HIF1α is inhibited, leading to a strong accumulation of HIF1α[4].
In addition, changes in the nucleotide sequence or expression of the HIF1α subunit are associated with the development of various diseases[2]. HIF1α polymorphisms, such as rs11549465 (Pro582Ser) and rs2057482, may impair the response to tissue hypoxia and influence the clinical manifestations of coronary atherosclerosis by affecting HIF1α subunit degradation and HIF1α mRNA stability[8]. The rs11549467 polymorphism is also important for HIF1α subunit stability[5]. The HIF1α rs2057482 polymorphism is a risk factor for the development of premature coronary heart disease[5]. These variations may influence the tissue response to hypoxia and affect the clinical manifestations of coronary atherosclerosis[8].
Cardiovascular diseases are prone to ischemic injury[2]. In these diseases, such as atherosclerosis and myocardial infarction, the oxygen supply to cells is reduced owing to impaired blood flow, eventually leading to tissue hypoxia[6], and cardiac hypoxia or ischemia[1]. Mammalian cells respond quickly and adapt to hypoxic conditions[2]. HIF1α plays a significant role in this process and confers cardioprotective effects to deoxygenated myocardium[2]. In humans, HIF1α insufficiency may correlate in part with congenital heart abnormalities[9]. HIF1α directly regulates over 1000 genes in the human genome during hypoxia, most of which are expressed in a specific cell type[9]. It is important to emphasize that this regulation is not an indirect, but a direct effect of HIF1α.
HIF1α acts as a cellular oxygen sensor in cardiomyocytes[10]. Its overexpression in the heart during acute myocardial infarction leads to the upregulation of proangiogenic HIF1α target genes, resulting in reduced cardiac dysfunction and decreased cardiomyocyte apoptosis[7]. In addition, excessive levels of HIF1α promote the expression of heme oxygenase-1 (HO-1), which reduces the accumulation of reactive oxygen species[10]. Moreover, increased HIF1α expression suppresses the pro-apoptotic gene BCL2 interacting protein-3 (BNIP3) via the nuclear factor kappa B (NF-κB) protein. HIF1α expression increases during myocardial infarction and serves as a regulator of the cellular hypoxia response[10]. The Pro582Ser (rs11549465) polymorphism in HIF1α affects the response to ischemic cardiovascular diseases. Furthermore, inhibition of HIF1α or HIF1β expression in myocardial endothelial cells leads to a lack of acute cardioprotection after ischemic preconditioning[9]. Inhibition of HIF1α in the myocardium could either promote or impair cardiomyocyte apoptosis. Increased expression of HIF1α in myocardial infarction significantly reduces the size of the infarct and restores the typical histologic structure of the myocardium. In addition, overexpression of HIF1α reduces the oxidative stress load during myocardial infarction[10]. Increased HIF1α expression promotes NF-κB binding to the BNIP3 promoter, which reduces BNIP3 expression and BNIP3-mediated apoptotic activity in hypoxic cardiomyocytes[10]. The signaling pathways mediated by HIF1α and NF-κB show synergistic interaction to reduce cardiomyocyte apoptosis. Increased cardiac-specific HIF1α expression during myocardial infarction leads to differential regulation of HO-1 and BNIP3 expression by HIF1α and NF-κB[10], demonstrating the crucial role of these signaling pathways in cardioprotection. In addition, HIF1α influences the balance between glycolytic and oxidative metabolism, with elevated levels of HIF1α leading to the expression of genes responsible for glucose transporters and glycolytic enzymes[9]. As a result, expression of HIF1α has been shown to be sufficient to trigger reperfusion in the ischemic skeletal muscle. However, HIF1α expression was reduced in the hypoxic skin wounds of old diabetic mice[2]. HIF1α may have a protective, proangiogenic, and pathogenic effect during infarction as it regulates metabolic reprogramming leading to energy depletion[9].
Cardiac hypoxia is usually caused by myocardial ischemia, which occurs when the metabolic needs of the heart muscle are not met owing to insufficient oxygen supply[2]. Arterial stenosis-induced hypoxia promotes the expression of HIF1α, which in turn stimulates the production of angiogenic growth factors, leading to vascular remodeling and increased blood flow. HIF1α is essential for ischemic preconditioning as it reduces reactive oxygen species production, protecting the heart from injury[9]. Chronic disease and aging impede this response. HIF1α plays a multifaceted role in the pathophysiology of myocardial infarction. It may be protective by promoting angiogenesis or pathologic through maladaptive metabolic reprogramming[9].
Different genetic variations of HIF1α can potentially affect the risk of myocardial infarction by influencing numerous mechanisms. Cardiac ischemia induces strong HIF1α expression, which could stimulate the formation of new blood vessels near the coronary arteries. Variations in HIF1α may alter the risk of acute myocardial infarction by inhibiting the development of new blood vessels near the atherosclerotic plaques in the coronary arteries[8]. Certain polymorphisms of HIF1α have been associated with cardiovascular diseases, including rs11549465, rs10873142, rs2057482, rs11549467, rs41508050, rs2783778, and rs7148720[2]. Several studies have investigated the association between different polymorphisms of HIF1α and cardiovascular diseases. However, conflicting and controversial results have been reported, indicating both positive and negative associations between HIF1α variations and cardiovascular diseases[5,8].
Research has indicated a link between circadian and hypoxic molecular pathways. HIF1α acts as an oxygen sensor, whereas period circadian regulator 2 (PER2) acts as a light sensor[7]. In response to HIF1α, several circadian rhythm genes respond to changes in oxygen levels[4]. HIF1α is able to induce the expression of PER2 and cryptochrome circadian regulator 1 (CRY1)[1]. Stabilization of HIF1α by PER2 is necessary for myocardial adaptation to hypoxia[11]. HIF1α regulates the hypoxic response to myocardial infarction via the circadian rhythm and influences the expression of target genes[1]. The adaptation of cardiomyocytes to hypoxia, known as ischemic demand, makes them more resistant to infarction by expressing high levels of PER2 and HIF1α[11]. HIF1α, HIF1β, basic helix-loop-helix ARNT like 1 (BMAL1), and circadian locomotor output cycles kaput (CLOCK) are transcription factors that respond to physiological and environmental signals. In addition, HIF1α can regulate circadian rhythms, whereas circadian proteins have the ability to influence the HIF1α response[12]. Additionally, similar to BMAL1, HIF1α contains a basic helix-loop-helix - period-ARNT-single minded (bHLH-PAS) domain. Through this domain, it dimerizes with BMAL1 and stimulates the expression of target genes. Thus, HIF1α serves as a molecular link between oxygen levels and the circadian rhythm[13].
The HIF1α-BMAL1 heterodimer binds to the same E-box regions of target genes as the CLOCK-BMAL1 heterodimer and influences the expression of downstream genes such as PER2, CRY1, and HIF1α target genes[1] (Figure 1). HIF1α is associated with vascular inflammation and the progression of atherosclerosis, whereas CLOCK and BMAL1 can also promote HIF1α expression[1]. Furthermore, myocardial ischemia triggers pathways to improve oxygen delivery and, during hypoxia, PER2 interacts with HIF1α[13]. This occurs because PER2 stabilizes HIF1α via adenosine receptor A2B (ADORA2B), which is crucial for myocardial adaptation to hypoxia[14]. Additionally, daily rhythms are present in blood and tissue oxygenation, oxygen usage, and carbon dioxide release. Exposure to hypoxia leads to tissue- and time-specific changes in the expression of circadian clock genes. Myocardial tissue damage is associated with the time of day of infarction, suggesting a link between HIF1α and circadian regulation of infarction[15]. Severe hypoxia-induced outcomes, namely myocardial infarction, are associated with changes in circadian rhythm. The circadian rhythm plays a crucial role in fine-tuning hypoxic responses during pathological circumstances[15].
Mice lacking Per2 are unable to maintain the stability of the HIF1α subunit in the myocardium during hypoxia, leading to increased cardiomyocyte death during ischemia[1]. Furthermore, myocardial damage after myocardial infarction appears to be worse in mice lacking Per1 and Per2 than in wild-type mice[15]. Within the physiological range, the oxygen cycle appropriately synchronizes cellular circadian clocks through a HIF1α-dependent mechanism. A slight reduction in oxygen levels for a short period of time facilitates adaptation to the time changes after jet lag in wild-type mice, but not in HIF1α-null mice[4].
Hypoxia and changing oxygen levels affect the circadian rhythm through different mechanisms involving HIF1α[1]. The circadian rhythm protects the heart muscle from hypoxia-induced cell death[1].
The relationship between hypoxia and circadian molecular signaling pathways needs further clarification in many physiological and pathophysiological processes, as these pathways are evolutionarily conserved and allow cells to adapt to unfavorable environmental conditions. The timing of the experiment significantly influences the circadian rhythm and, subsequently, HIF1α levels, which are associated with the severity of cardiovascular diseases. Studying the use of molecular signaling pathways in tissues and how they are influenced by specific diseases, particularly in the context of cardiovascular disease, presents new therapeutic possibilities for the treatment of diseases with low oxygen availability, such as myocardial infarction.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Adam CA, Romania; Kawaguchi N, Japan S-Editor: Gong ZM L-Editor: A P-Editor: Guo X
| 1. | O'Connell EJ, Martinez CA, Liang YG, Cistulli PA, Cook KM. Out of breath, out of time: interactions between HIF and circadian rhythms. Am J Physiol Cell Physiol. 2020;319:C533-C540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Gladek I, Ferdin J, Horvat S, Calin GA, Kunej T. HIF1A gene polymorphisms and human diseases: Graphical review of 97 association studies. Genes Chromosomes Cancer. 2017;56:439-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Knutson AK, Williams AL, Boisvert WA, Shohet RV. HIF in the heart: development, metabolism, ischemia, and atherosclerosis. J Clin Invest. 2021;131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 4. | Adamovich Y, Ladeuix B, Golik M, Koeners MP, Asher G. Rhythmic Oxygen Levels Reset Circadian Clocks through HIF1α. Cell Metab. 2017;25:93-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 186] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 5. | López-Reyes A, Rodríguez-Pérez JM, Fernández-Torres J, Martínez-Rodríguez N, Pérez-Hernández N, Fuentes-Gómez AJ, Aguilar-González CA, Alvarez-León E, Posadas-Romero C, Villarreal-Molina T, Pineda C, Vargas-Alarcón G. The HIF1A rs2057482 polymorphism is associated with risk of developing premature coronary artery disease and with some metabolic and cardiovascular risk factors. The Genetics of Atherosclerotic Disease (GEA) Mexican Study. Exp Mol Pathol. 2014;96:405-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Abe H, Semba H, Takeda N. The Roles of Hypoxia Signaling in the Pathogenesis of Cardiovascular Diseases. J Atheroscler Thromb. 2017;24:884-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 7. | Bartman CM, Eckle T. Circadian-Hypoxia Link and its Potential for Treatment of Cardiovascular Disease. Curr Pharm Des. 2019;25:1075-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Hlatky MA, Quertermous T, Boothroyd DB, Priest JR, Glassford AJ, Myers RM, Fortmann SP, Iribarren C, Tabor HK, Assimes TL, Tibshirani RJ, Go AS. Polymorphisms in hypoxia inducible factor 1 and the initial clinical presentation of coronary disease. Am Heart J. 2007;154:1035-1042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol. 2014;76:39-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 416] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 10. | Datta Chaudhuri R, Banik A, Mandal B, Sarkar S. Cardiac-specific overexpression of HIF-1α during acute myocardial infarction ameliorates cardiomyocyte apoptosis via differential regulation of hypoxia-inducible pro-apoptotic and anti-oxidative genes. Biochem Biophys Res Commun. 2021;537:100-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Škrlec I, Marić S, Včev A. Myocardial infarction and circadian rhythm [Internet]. In: Angelos Tsipis. Visions of Cardiomyocyte - Fundamental Concepts of Heart Life and Disease. London, UK: IntechOpen; 2020: 21-35. Available from: https://www.intechopen.com/online-first/myocardial-infarction-and-circadian-rhythm. [Cited in This Article: ] |
| 12. | Egg M, Köblitz L, Hirayama J, Schwerte T, Folterbauer C, Kurz A, Fiechtner B, Möst M, Salvenmoser W, Sassone-Corsi P, Pelster B. Linking oxygen to time: the bidirectional interaction between the hypoxic signaling pathway and the circadian clock. Chronobiol Int. 2013;30:510-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Škrlec I. Circadian system microRNAs - Role in the development of cardiovascular diseases. Adv Protein Chem Struct Biol. 2023;137:225-267. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 14. | Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 15. | Wu Y, Tang D, Liu N, Xiong W, Huang H, Li Y, Ma Z, Zhao H, Chen P, Qi X, Zhang EE. Reciprocal Regulation between the Circadian Clock and Hypoxia Signaling at the Genome Level in Mammals. Cell Metab. 2017;25:73-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |