Copyright
©The Author(s) 2015.
World J Biol Chem. Aug 26, 2015; 6(3): 95-109
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.95
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.95
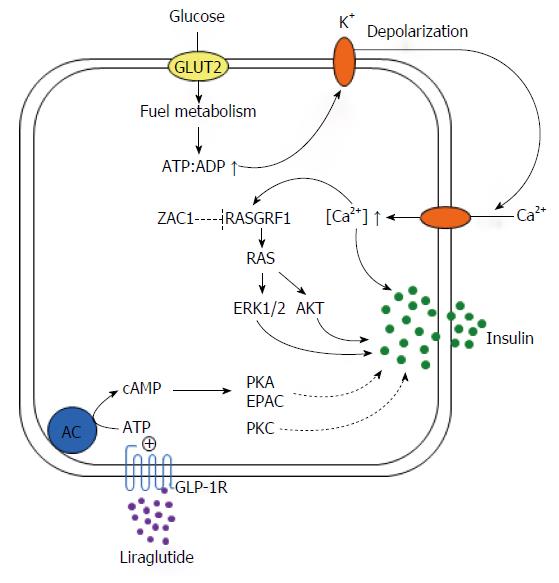
Figure 2 Roles for RAS protein-specific guanine nucleotide-releasing factor 1 in insulin secretion and transient neonatal diabetes mellitus type 1.
Glucose is the main stimulus for insulin secretion. Once transported into pancreatic β-cells by glucose transporter 2 (GLUT2), metabolisation of glucose results in the production of ATP. A subsequent rise in the ATP:ADP ratio drives closure of ATP-regulated K+ channels and accumulation of intracellular K+. Heightened K+ concentrations lead to depolarization of the plasma membrane and promote opening of voltage-dependent Ca2+ channels with subsequent influx of Ca2+ and increases in free cytoplasmatic Ca2+. This event stimulates exocytosis of insulin from the insulin-storing secretory granules through different routes including activation of the Ca2+-calmodulin activated RAS protein-specific guanine nucleotide-releasing factor 1 (RASGRF1), a direct ZAC1 target gene. In its activated state the small G-protein RAS couples to ERK1/2 and PI3K/AKT signaling among other downstream effectors, which jointly enhance the exocytotic process of insulin secretion. ZAC1 overexpression in transient neonatal diabetes mellitus type 1 (TNDM1) is predicted to directly repress RASGRF1 and consequently GSIS. Contrarily, PKA signaling is undisturbed in TNDM1 as well as the potentiating effects of the GLP-1R agonist liraglutide on GSIS.
- Citation: Hoffmann A, Spengler D. Role of ZAC1 in transient neonatal diabetes mellitus and glucose metabolism. World J Biol Chem 2015; 6(3): 95-109
- URL: https://www.wjgnet.com/1949-8454/full/v6/i3/95.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.95