Copyright
©The Author(s) 2015.
World J Biol Chem. Aug 26, 2015; 6(3): 95-109
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.95
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.95
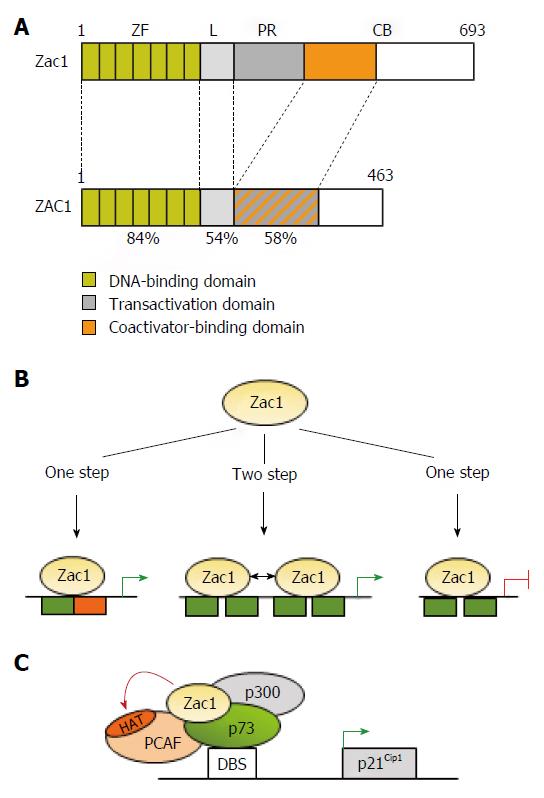
Figure 1 Transcriptional activities of Zac1.
A: Schematic drawing of Zac1 proteins. Domains are boxed and numbers refer to amino acids and percentage of homology between mouse and human. The N-terminal DNA binding domain is highly conserved between human and mice and comprises seven canonical C2H2-type zinc fingers (ZF). The linker domain (L) in conjunction with the proline-rich region (PR) confers transactivation in mice, which is further enhanced through the C-terminus’s coactivator binding (CB) domain. Contrarily, transactivation and coactivator recruitment jointly localize to ZAC1’s C-terminus; B: Zac1 recognizes different DNA-elements to confer transcriptional regulation. Monomeric Zac1 binding to GC-rich palindromes (left) or dimerization at G/C rich direct repeat elements (middle) results in transactivation. On the other hand, monomeric Zac1 binding to G/C rich half sites causes repression (right); C: Zac1 coactivation of p73. Following recognition of its DNA-binding site (DBS), the transcription factor p73 recruits Zac1 together with the general coactivator p300 and PCAF to the p21Cip1 promoter during early neural differentiation. Due to its scaffolding function, Zac1’s zinc fingers stabilize this interaction and enhance additionally PCAF’s histone acetyltransferase (HAT) activity. This event enhances histone acetylation at the p21Cip1 promoter and subsequent transcription.
- Citation: Hoffmann A, Spengler D. Role of ZAC1 in transient neonatal diabetes mellitus and glucose metabolism. World J Biol Chem 2015; 6(3): 95-109
- URL: https://www.wjgnet.com/1949-8454/full/v6/i3/95.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.95