Copyright
©The Author(s) 2015.
World J Biol Chem. Aug 26, 2015; 6(3): 83-94
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.83
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.83
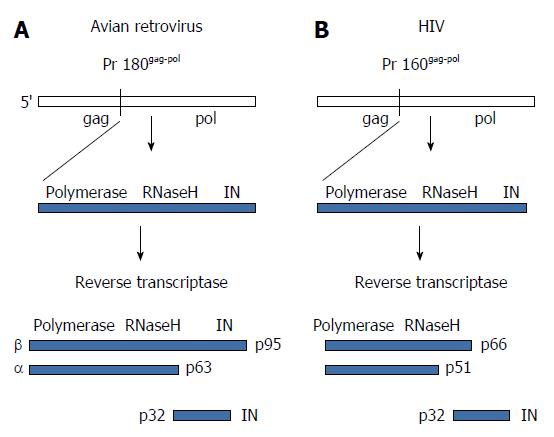
Figure 3 Structural organization and proteolytic processing of reverse transcriptase and integrase.
A: The precursor Gag-Pol protein is processed by the viral protease into an intermediate polyprotein. The avian polyprotein exists as a dimer of the β subunit that is processed into the active αβ subunits (RT) and integrase (IN). The α subunit contains the active polymerase and RNaseH sites while β retains IN residues. RT does not possess integration capabilities; B: Cleavage of the human immunodeficiency virus (HIV) polyprotein produces the active dimeric RT and IN. The HIV p66 subunit possesses the polymerase and RNaseH active sites. RT: Reverse transcriptase.
- Citation: Grandgenett DP, Pandey KK, Bera S, Aihara H. Multifunctional facets of retrovirus integrase. World J Biol Chem 2015; 6(3): 83-94
- URL: https://www.wjgnet.com/1949-8454/full/v6/i3/83.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.83