Copyright
©The Author(s) 2015.
World J Biol Chem. Aug 26, 2015; 6(3): 249-264
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.249
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.249
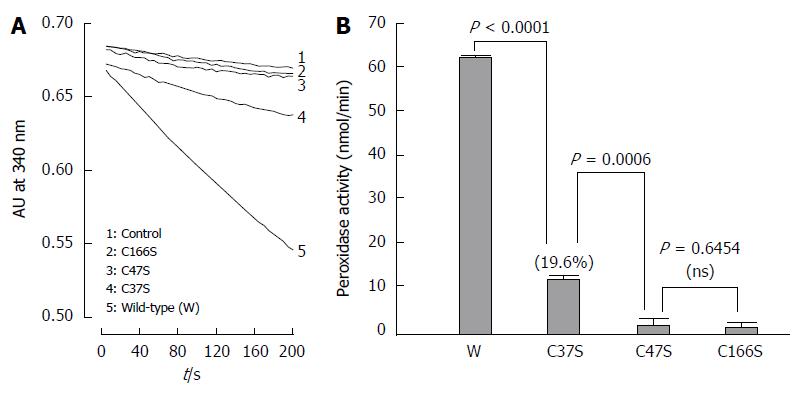
Figure 8 Trx system-supported alkyl hydroperoxide peroxidase activity of the wild-type and mutant AhpC_H1 proteins.
A: Peroxidase reaction was initiated by adding 2 μmol/L of the wild-type (W) or C37S, C47S, and C166S mutated AhpC_H1 enzymes to the reaction mixture containing 50 mmol/L Hepes-NaOH, pH 7.4, 1 mmol/L EDTA, 100 nmol/L of E. coli Trx reductase, 5 μmol/L of E. coli Trx, and 0.14 mM NADPH; the activity was continuously monitored by the decrease in 340-nm absorbance at 25 °C; B: Initial peroxidase activity rate of the wild-type and mutant AhpC_H1. The data are expressed as the mean ± standard error of three independent measurements.
-
Citation: Cha MK, Bae YJ, Kim KJ, Park BJ, Kim IH. Characterization of two alkyl hydroperoxide reductase C homologs alkyl hydroperoxide reductase C_H1 and alkyl hydroperoxide reductase C_H2 in
Bacillus subtilis . World J Biol Chem 2015; 6(3): 249-264 - URL: https://www.wjgnet.com/1949-8454/full/v6/i3/249.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.249