Copyright
©The Author(s) 2015.
World J Biol Chem. Aug 26, 2015; 6(3): 249-264
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.249
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.249
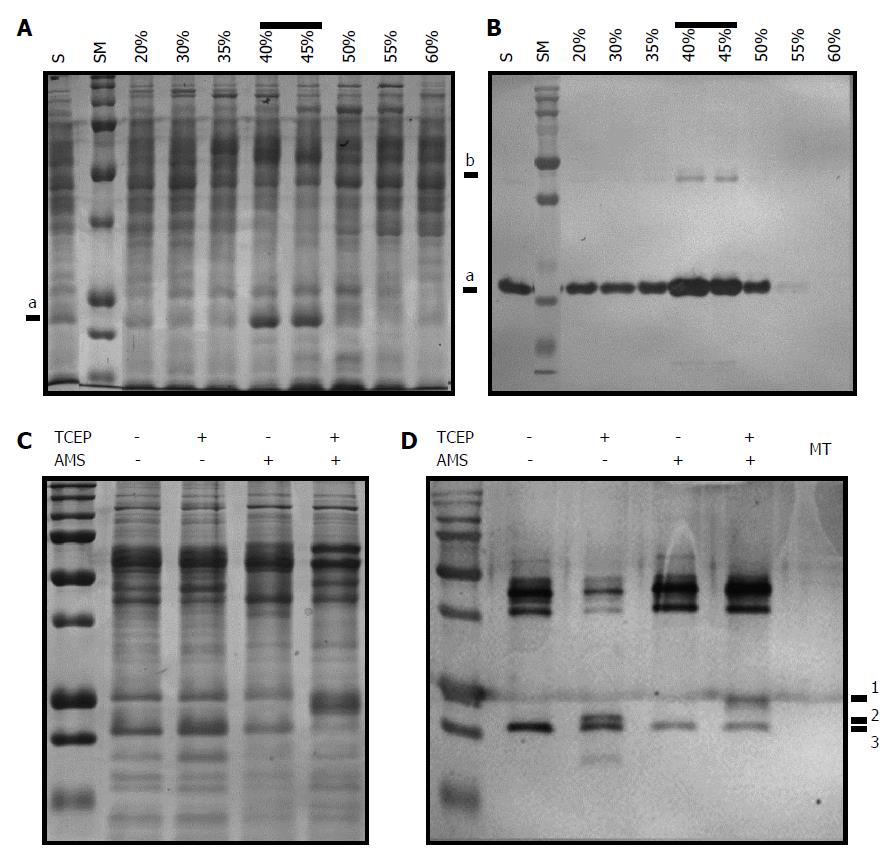
Figure 5 Identification of intramolecular disulfide linkage in AhpC_H1 from B.
subtilis. A, B: Soluble fraction of B. subtilis overnight cultures was precipitated with 30%, 35%, 40%, 45%, 50%, 55%, and 60% ammonium sulfate. After dialysis of solubilized precipitates, 20 μg of each fraction was separated in 14% reducing SDS-PAGE gel, and stained with Coomassie brilliant blue (A) or processed by western blotting using anti-AhpC_H1 antibody (for western blotting, 1 μg protein was separated). Correlation of band intensities in (A) and (B) indicates that the major band marked as a is an AhpC_H1 monomer; b marks an AhpC_H1 dimer; C: Proteins (20 μg) of the 40% fraction were incubated in the presence or absence of 2 mM TCEP, reacted with 5 mmol/L of AMS for 30 min at 30 °C, separated by non-reducing SDS-PAGE, and stained with Coomassie Brilliant Blue; D: Similarly treated proteins (100 ng) were subjected to western blotting. Triple bands (1, 2, and 3) around the position of the monomer indicate AMS modification of Сys residues after the reduction by TCEP. MT, AhpC_H1-deficient B. subtilis; the absence of the AhpC_H1 band indicates antibody specificity. SM, molecular weight markers (15, 20, 25, 37, 50, 75, 100, 150, 250 kDa).
-
Citation: Cha MK, Bae YJ, Kim KJ, Park BJ, Kim IH. Characterization of two alkyl hydroperoxide reductase C homologs alkyl hydroperoxide reductase C_H1 and alkyl hydroperoxide reductase C_H2 in
Bacillus subtilis . World J Biol Chem 2015; 6(3): 249-264 - URL: https://www.wjgnet.com/1949-8454/full/v6/i3/249.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.249