Copyright
©The Author(s) 2015.
World J Biol Chem. Aug 26, 2015; 6(3): 249-264
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.249
Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.249
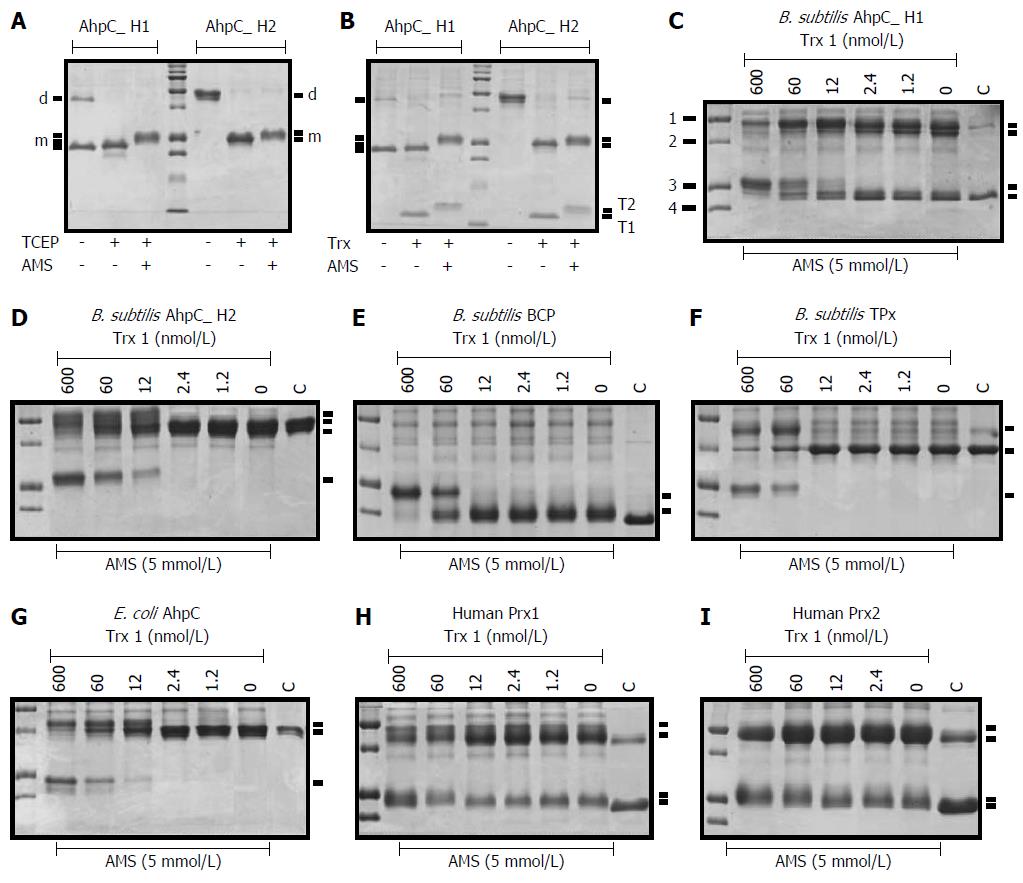
Figure 4 Non-reducing SDS-PAGE of peroxiredoxins reduced with the Trx system.
(A, B) AhpC_H1 and AhpC H2 were incubated without or with 2 mnol/L TCEP (A) or Trx system (B) for 30 min at 30 °C, followed by incubation with AMS for 30 min at 30 °C. The reaction was terminated by adding SDS sample buffer, and proteins were separated in 16% non-reducing SDS-PAGE gels. Protein migration pattern in A is the same as in B, suggesting that the Trx system reduced disulfide bonds in both proteins; d and m mark the position of dimeric and monomeric, respectively, AhpC_H1 and AhpC_H2. T1 and T2 denote the oxidized and reduced/modified Trx 1 proteins, respectively. Middle lanes show molecular weight markers (10, 15, 20, 25, 37, 50, 75, 100, 150, and 250 kDa). Trx, the Trx system; (C-F) Conversion of different Prxs from the oxidized to reduced form by the Trx system. 1, 2, 3, and 4 indicate molecular weight markers of 50, 37, 25, and 20 kDa, respectively.
-
Citation: Cha MK, Bae YJ, Kim KJ, Park BJ, Kim IH. Characterization of two alkyl hydroperoxide reductase C homologs alkyl hydroperoxide reductase C_H1 and alkyl hydroperoxide reductase C_H2 in
Bacillus subtilis . World J Biol Chem 2015; 6(3): 249-264 - URL: https://www.wjgnet.com/1949-8454/full/v6/i3/249.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.249