Copyright
©The Author(s) 2015.
World J Biol Chem. Feb 26, 2015; 6(1): 1-15
Published online Feb 26, 2015. doi: 10.4331/wjbc.v6.i1.1
Published online Feb 26, 2015. doi: 10.4331/wjbc.v6.i1.1
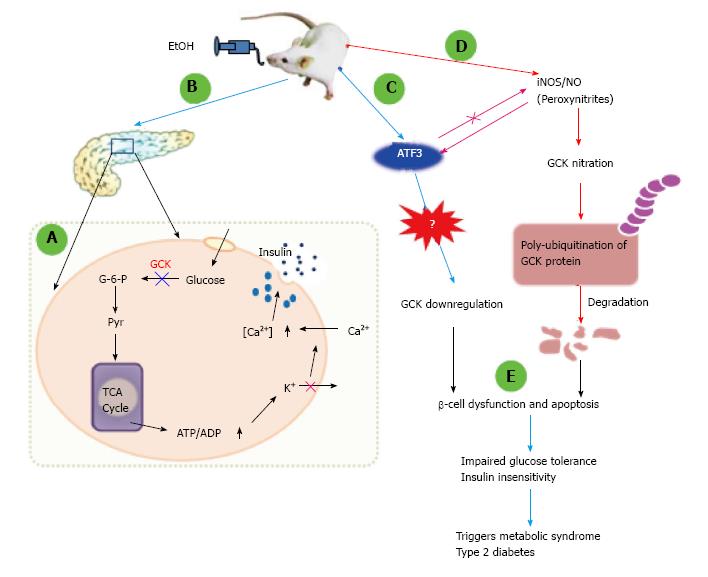
Figure 2 Proposed model by which peroxynitrite-mediated activating transcription factor 3 and glucokinase nitration in ethanol-fed mice trigger metabolic syndrome through pancreatic β-cell dysfunction and apoptosis.
A: Under normal conditions, when glucose is taken up by pancreatic β-cells, it is converted to glucose-6-phosphate by glucokinase. Pyruvate transferred into the mitochondria activates the TCA cycle, which produces ATP. Increased ATP/ADP ratios prevent potassium efflux via inhibiting potassium channels and opening calcium channels. Increased intracellular calcium levels activate insulin maturation and its secretion; B: In mice fed with a 5% (v/v) ethanol diet for 8 wk, glucokinase (GCK) protein expression was significantly decreased in pancreatic tissues and isolated islet cells; C: Ethanol-fed mice showed high expression of activating transcription factor (ATF) 3. ATF3 is regulated by the ethanol metabolism pathway because ethanol-induced ATF3 was significantly decreased by 4-methylpyrazole, an inhibitor of cytochrome P450 2E1. ATF3 expression is regulated by the peroxynitrite-dependent pathway, but ATF3 did not affect the ethanol-mediated production of peroxynitrite. Despite this discrepancy, the C-terminal domain of ATF3 is involved in the downregulation of GCK. However, the role of ATF3 and the exact regulatory mechanisms involved in GCK downregulation are still not fully understood; D: Ethanol-produced peroxynitrite significantly increased GCK nitration on tyrosine residues; this effect was abolished by an iNOS inhibitor, L-NMMA, or the peroxynitrite scavengers, uric acid and deferoxamine. Interestingly, the nitrated GCK was more susceptible to ubiquitination than were the native proteins of control cells, making it vulnerable to degradation; E: Cells with downregulated GCK expression lack the ability to synthesize insulin and eventually progress to apoptosis. Mice lacking the ability to produce insulin may easily develop metabolic syndrome with impaired glucose tolerance and insulin insensitivity, resulting in type 2 diabetes.
- Citation: Kim JY, Lee DY, Lee YJ, Park KJ, Kim KH, Kim JW, Kim WH. Chronic alcohol consumption potentiates the development of diabetes through pancreatic β-cell dysfunction. World J Biol Chem 2015; 6(1): 1-15
- URL: https://www.wjgnet.com/1949-8454/full/v6/i1/1.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i1.1