Copyright
©2014 Baishideng Publishing Group Inc.
World J Biol Chem. Aug 26, 2014; 5(3): 308-320
Published online Aug 26, 2014. doi: 10.4331/wjbc.v5.i3.308
Published online Aug 26, 2014. doi: 10.4331/wjbc.v5.i3.308
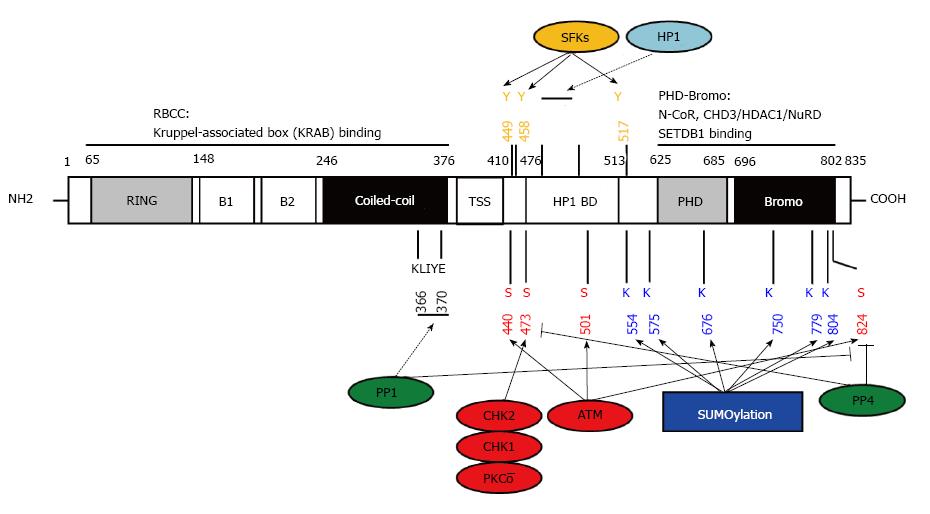
Figure 2 KAP1 structure, post-translational modifications and interacting proteins.
KAP1 has multi-domains for protein-protein interaction and post-translational modification. RBCC: RING-B1-B2-coiled-coil; TSS: TIF signature sequence; HP1 BD: HP1 binding domain; PHD: Plant homeo domain. Numbers represent the sequence of amino acids; Blue: SUMOylation sites; Red: Serine phosphorylation sites targeted by the indicated kinases (shown in red) or antagonized by phosphatases (shown in green); Orange: Tyrosine phosphorylation sites targeted by the indicated kinase family; KLIYF: PP1 binding site; PxVxL: HP1 binding site; Dotted lines: Protein-protein interaction; SFKs: Src family kinases; HP1: Heterochromatin-associated protein 1; ATM: Ataxia-telangiectasia mutated; PP1: Protein phosphatase 1; CHD3/NuRD: Chromodomain helicase DNA binding protein 3/nucleosome remodeling deacetylase; HDAC: Histone deacetylase.
- Citation: Cheng CT, Kuo CY, Ann DK. KAPtain in charge of multiple missions: Emerging roles of KAP1. World J Biol Chem 2014; 5(3): 308-320
- URL: https://www.wjgnet.com/1949-8454/full/v5/i3/308.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i3.308