Copyright
©2014 Baishideng Publishing Group Inc.
World J Biol Chem. May 26, 2014; 5(2): 254-268
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.254
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.254
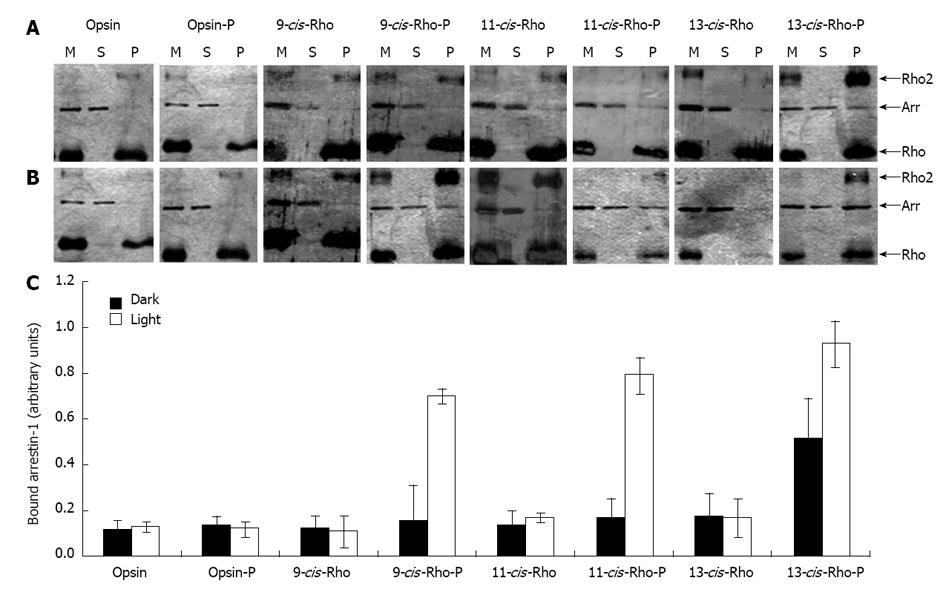
Figure 7 Ability of arrestin-1 to interact with the reconstituted rhodopsin and rhodopsin analogues.
Arrestin-1 was combined with membranes containing opsin, phosphorylated opsin (opsin-P), isorhodopsin (9-cis-Rho), phosphorylated isorhodopsin (9-cis-Rho-P), rhodopsin (11-cis-Rho), phosphorylated rhodopsin (11-cis-Rho-P), 13-cis-retinal-rhodopsin (13-cis-Rho), and phosphorylated 13-cis-retinal-rhodopsin (13-cis-Rho-P), under dark (Panel A) and light (Panel B) conditions. The mixtures (M) were centrifuged and aliquots of each mixture and of the resulting supernatants (S) and pellets (P) were separated by SDS-PAGE. Gels were colored by silver staining. In Panel C, the amount of arrestin-1 that interacted with the phosphorylated pigments in the pellet fraction was quantified by densitometry. Mean ± SD of three independent experiments are reported. Differences with P-values < 0.05 were considered significant Arrows indicate the migration of rhodopsin (Rho), arrestin-1 (Arr), and rhodopsin dimers (Rho2).
- Citation: Araujo NA, Sanz-Rodríguez CE, Bubis J. Binding of rhodopsin and rhodopsin analogues to transducin, rhodopsin kinase and arrestin-1. World J Biol Chem 2014; 5(2): 254-268
- URL: https://www.wjgnet.com/1949-8454/full/v5/i2/254.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i2.254