Copyright
©2014 Baishideng Publishing Group Inc.
World J Biol Chem. May 26, 2014; 5(2): 240-253
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.240
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.240
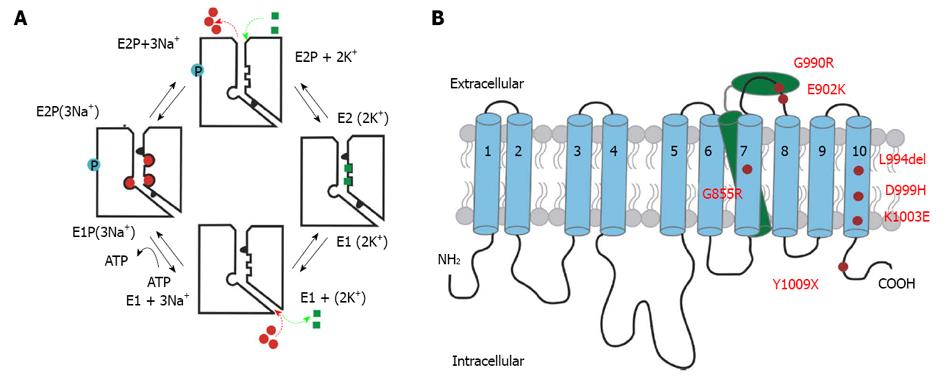
Figure 1 Reaction mechanism and structural detail of the Na+/K+-ATPase.
A: Schematic reaction cycle of one Na+/K+-ATPase pump molecule. The cytosolic side is shown at the bottom of each molecule depicted with an ion pathway to the right, whereas the extracellular side is set at the top. Na+ ions are shown as red circles, and K+ ion are shown as green squares. Blue circles depict the phosphorylated state; B: Simplified structure of the Na+/K+-ATPase indicating FHM2/SHM mutation positions studied in this work. The α-subunit is composed of ten transmembrane domains (blue). The N- and C-terminus are located intracellularly. The β-subunit comprises only one transmembrane domain (green) and a large ectodomain with several glycosylation sites. FHM2/SHM mutations are marked in red.
- Citation: Spiller S, Friedrich T. Functional analysis of human Na+/K+-ATPase familial or sporadic hemiplegic migraine mutations expressed in Xenopus oocytes. World J Biol Chem 2014; 5(2): 240-253
- URL: https://www.wjgnet.com/1949-8454/full/v5/i2/240.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i2.240