Copyright
©2014 Baishideng Publishing Group Inc.
World J Biol Chem. May 26, 2014; 5(2): 224-230
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.224
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.224
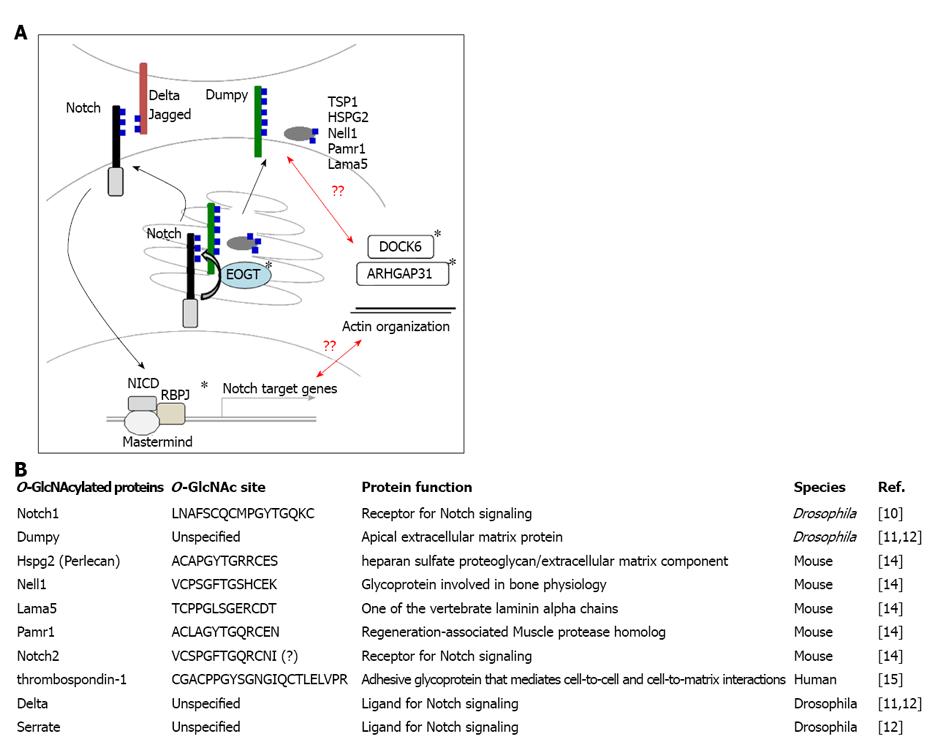
Figure 1 Extracellular O-linked β-N-acetylglucosamine.
A: The O-linked β-N-acetylglucosamine (O-GlcNAc)ylation of extracellular protein domains is a newly identified translational modification of epidermal growth factor (EGF) domains, including Notch, HSPG2, Pamr1, and Lama5. Extracellular O-GlcNAc is mediated by EOGT in the endoplasmic reticulum (ER). Mutations in EOGT were recently identified in patients with Adams-Oliver syndrome (AOS). The role of EOGT in the pathogenesis of AOS is currently unknown. Given that RBPJ, a transcriptional factor for Notch signaling, is a causative gene for AOS, O-GlcNAcylation of Notch receptors by EOGT might regulate Notch receptor trafficking or Notch-ligand interactions. ARHGAP31 or DOCK6, another causative gene for AOS, affects the actin cytoskeleton by regulating Cdc42 and Rac1 activity. Thus, another possibility is that the O-GlcNAcylation of unidentified cell adhesion molecules by EOGT affects actin dynamics. It should be noted, however, that Dumpy homologues are not present in mammals. The O-GlcNAcylation of Notch ligands was reported in Drosophila. The causative genes for AOS are shown by asterisks; B: Summary of proteins with extracellular O-GlcNAc identified to date.
-
Citation: Ogawa M, Furukawa K, Okajima T. Extracellular
O -linked β-N -acetylglucosamine: Its biology and relationship to human disease. World J Biol Chem 2014; 5(2): 224-230 - URL: https://www.wjgnet.com/1949-8454/full/v5/i2/224.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i2.224