Copyright
©2014 Baishideng Publishing Group Inc.
World J Biol Chem. May 26, 2014; 5(2): 180-203
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.180
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.180
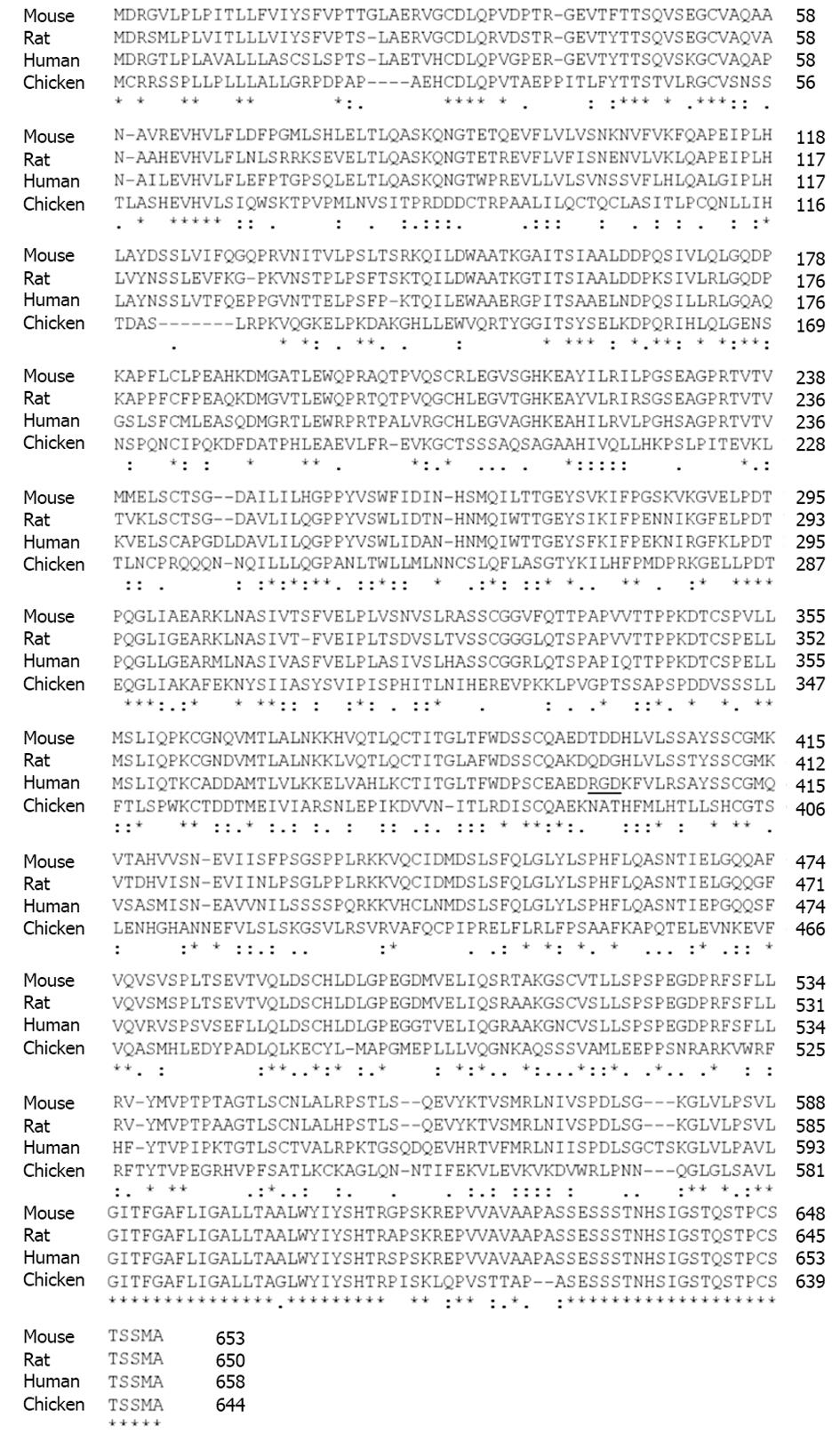
Figure 3 Sequence alignment of endoglin from different species.
The protein sequences of rat, mouse, human, and chicken endoglin were aligned using the ClustalW2 tool (http://www.expasy.org/genomics/sequence_alignment). Sequences of mouse (NP_031958), rat (AAS67893), human (NP_001108225) and chicken (AAT84715) were taken from the GenBank (http://www.ncbi.nlm.nih.gov/). The Arginine-Glycin-Aspartic acid sequence in human endoglin (aa 399-aa 401) is underlined. Fully conserved aa in endoglin are marked by asterisk (*), positions that carry aa with strongly similar properties by a colon (:) and positions with weakly similar properties by a period (.), respectively. Please note that the highest degree of homology is found at the C-terminal regions that encompass the cytosolic part of endoglin.
- Citation: Meurer SK, Alsamman M, Scholten D, Weiskirchen R. Endoglin in liver fibrogenesis: Bridging basic science and clinical practice. World J Biol Chem 2014; 5(2): 180-203
- URL: https://www.wjgnet.com/1949-8454/full/v5/i2/180.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i2.180