Copyright
©2012 Baishideng Publishing Group Co.
World J Biol Chem. May 26, 2012; 3(5): 98-109
Published online May 26, 2012. doi: 10.4331/wjbc.v3.i5.98
Published online May 26, 2012. doi: 10.4331/wjbc.v3.i5.98
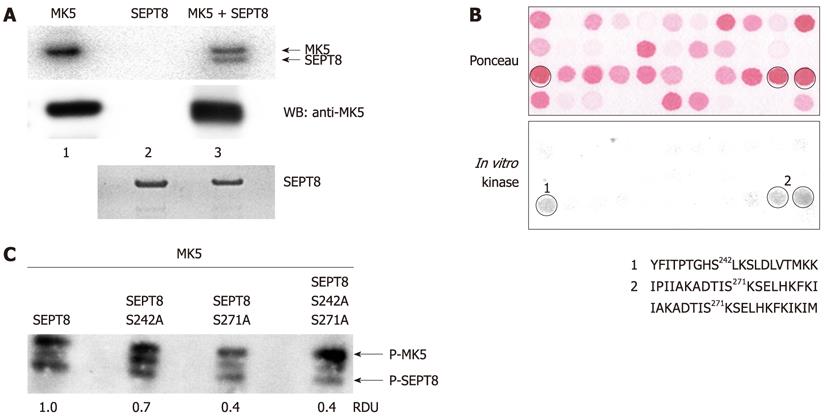
Figure 3 Mitogen-activated protein kinase-activated protein kinase 5 phosphorylates septin 8 in vitro.
A: In vitro kinase assay on recombinant SEPT8. Glutathione S-transferase (GST)-SEPT8 fusion protein was purified from Escherichia coli and the GST moiety was removed by thrombin. SEPT8 was incubated with activated mitogen-activated protein kinase-activated protein kinase 5 (MK5) for 30 min at 30 °C in the presence of [γ-32P] ATP. Proteins were separated by SDS-PAGE and phosphorylation was visualized by autoradiography (upper panel). Loading control for MK5 (middle panel) and SEPT8 (lower panel) was performed by western blotting with anti-PRAK and anti-SEPT8 antibodies, respectively. Lane 1: Recombinant activated MK5; lane 2: Purified SEPT8 protein; lane 3: recombinant MK5 and purified SEPT8; B: SEPT8 peptide array was subjected to in vitro phosphorylation by activated MK5. Each spot represents a 20-mer peptide fragment of SEPT8. The peptide fragments constitute the complete SEPT8 protein. The sequential peptide has a 17-amino acid overlap with the previous peptide. Several spots with peptides were detected by autoradiography (lower panel). Ser-242 and Ser-271 with highest prediction score 0.994 and 0.944, respectively by Netphos software, were chosen as possible phosphorylation sites in the peptide sequences. The upper panel represents Ponceau staining of the peptide array membrane used. The sequences of the peptides representing the positive spots are shown; C: In vitro phosphorylation of wild-type SEPT8, and SEPT8 mutants carrying a single amino acid substitution (Ser-242 into Ala or Ser-271 into Ala, respectively) or the double amino acid substitution Ser-242 and Ser-271 into Ala. The upper band represents autophosphorylated MK5, while the lower band is phosphorylated SEPT8. Relative densitometry units (RDU) of the bands representing phosphorylated SEPT8 are shown. The value obtain for wild-type SEPT8 was arbitrary set as 1.0 and the other values were related to this.
-
Citation: Shiryaev A, Kostenko S, Dumitriu G, Moens U. Septin 8 is an interaction partner and
in vitro substrate of MK5. World J Biol Chem 2012; 3(5): 98-109 - URL: https://www.wjgnet.com/1949-8454/full/v3/i5/98.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v3.i5.98