Copyright
©2012 Baishideng Publishing Group Co.
World J Biol Chem. May 26, 2012; 3(5): 98-109
Published online May 26, 2012. doi: 10.4331/wjbc.v3.i5.98
Published online May 26, 2012. doi: 10.4331/wjbc.v3.i5.98
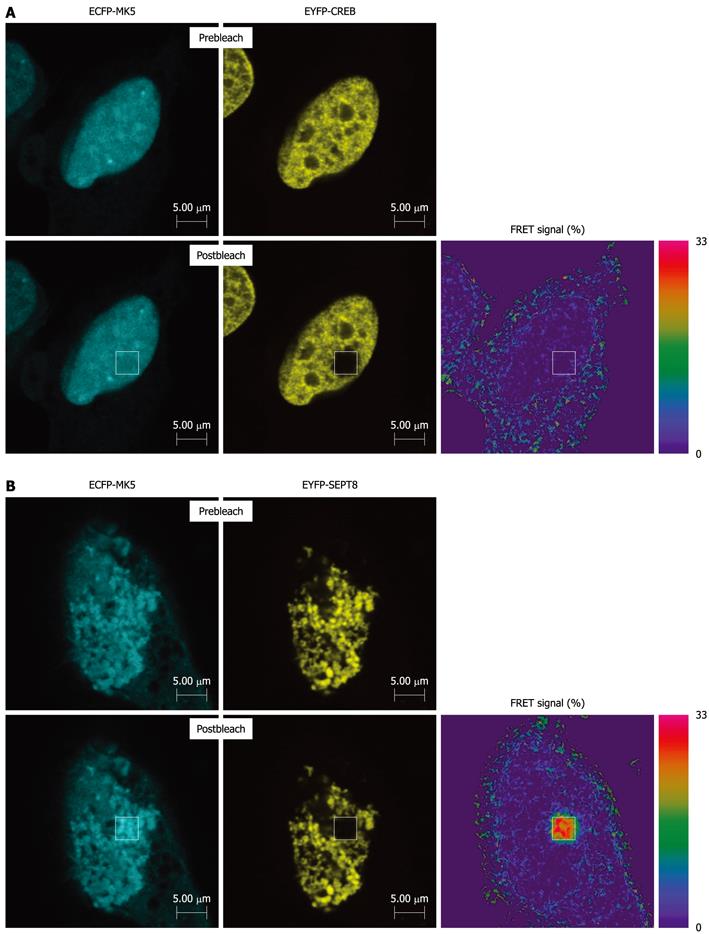
Figure 2 Fluorescence resonance energy transfer analysis shows interaction between mitogen-activated protein kinase-activated protein kinase 5 and SEPT8.
(A) HeLa cells were cotransfected with expression plasmids for heat shock protein (CFP)-tagged mitogen-activated protein kinase-activated protein kinase 5 (MK5) and yellow fluorescent protein (YFP)-tagged CREB or (B) with expression vectors for CFP-tagged MK5 and YFP-tagged SEPT8. Twenty-four hours after transfection, cells were fixed and fluorescence resonance energy transfer (FRET) analysis was carried out. CFP and YFP were excited with separate laser channels of 458 and 514 nm, respectively. Emission fluorescence intensity data were obtained at 465-500 nm (CFP) and 525-600 nm (YFP). CFP and YFP emission signals were captured before and after 50% photobleaching YFP. FRET is indicated as the relative increase in CFP emission following YFP photobleaching.
-
Citation: Shiryaev A, Kostenko S, Dumitriu G, Moens U. Septin 8 is an interaction partner and
in vitro substrate of MK5. World J Biol Chem 2012; 3(5): 98-109 - URL: https://www.wjgnet.com/1949-8454/full/v3/i5/98.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v3.i5.98