Copyright
©2012 Baishideng Publishing Group Co.
World J Biol Chem. Mar 26, 2012; 3(3): 53-60
Published online Mar 26, 2012. doi: 10.4331/wjbc.v3.i3.53
Published online Mar 26, 2012. doi: 10.4331/wjbc.v3.i3.53
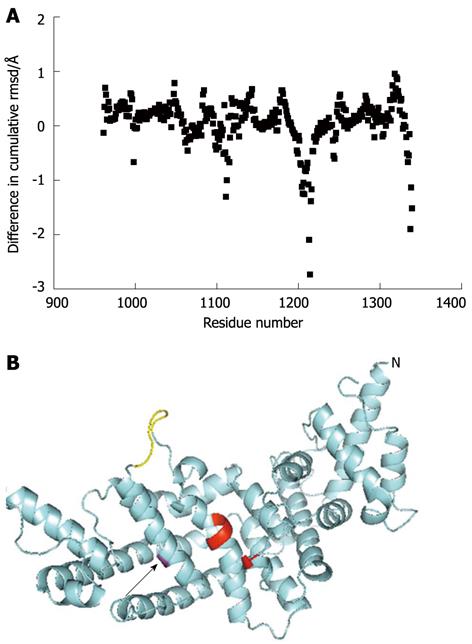
Figure 5 Predicted structural consequences of the M1231I, cancer associated, mutation.
A: The predicted effects on backbone flexibility as determined by FIRST/FRODAN (see Materials and Methods) are plotted as the difference in cumulative flexibility between the M1231I variant and wild type. Thus, a negative value represents in loss in flexibility of the variant compared to wild type. The greatest predicted rigidification occurs around residue 1214 and is indicated with an arrow; B: A model of the GAP-related domain of the M1231I variant is shown in cyan, with Ile-1231 in magenta and indicated with an arrow. The N-terminus of the fragment is marked (N) and the C-terminus can be seen close to this in space. Key residues predicted to be involved in CDC42 interaction (Thr-1046 and Tyr-1192 to Arg-1194) are shown in red. A loop (Ser-1212 to Leu-1217) predicted to be more rigid in the variant compared to the wild type is shown in yellow. It links the helix containing residue 1231 and part of the CDC42 binding site.
- Citation: Elliott SF, Allen G, Timson DJ. Biochemical analysis of the interactions of IQGAP1 C-terminal domain with CDC42. World J Biol Chem 2012; 3(3): 53-60
- URL: https://www.wjgnet.com/1949-8454/full/v3/i3/53.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v3.i3.53