Copyright
©The Author(s) 2023.
World J Biol Chem. Oct 17, 2023; 14(5): 84-98
Published online Oct 17, 2023. doi: 10.4331/wjbc.v14.i5.84
Published online Oct 17, 2023. doi: 10.4331/wjbc.v14.i5.84
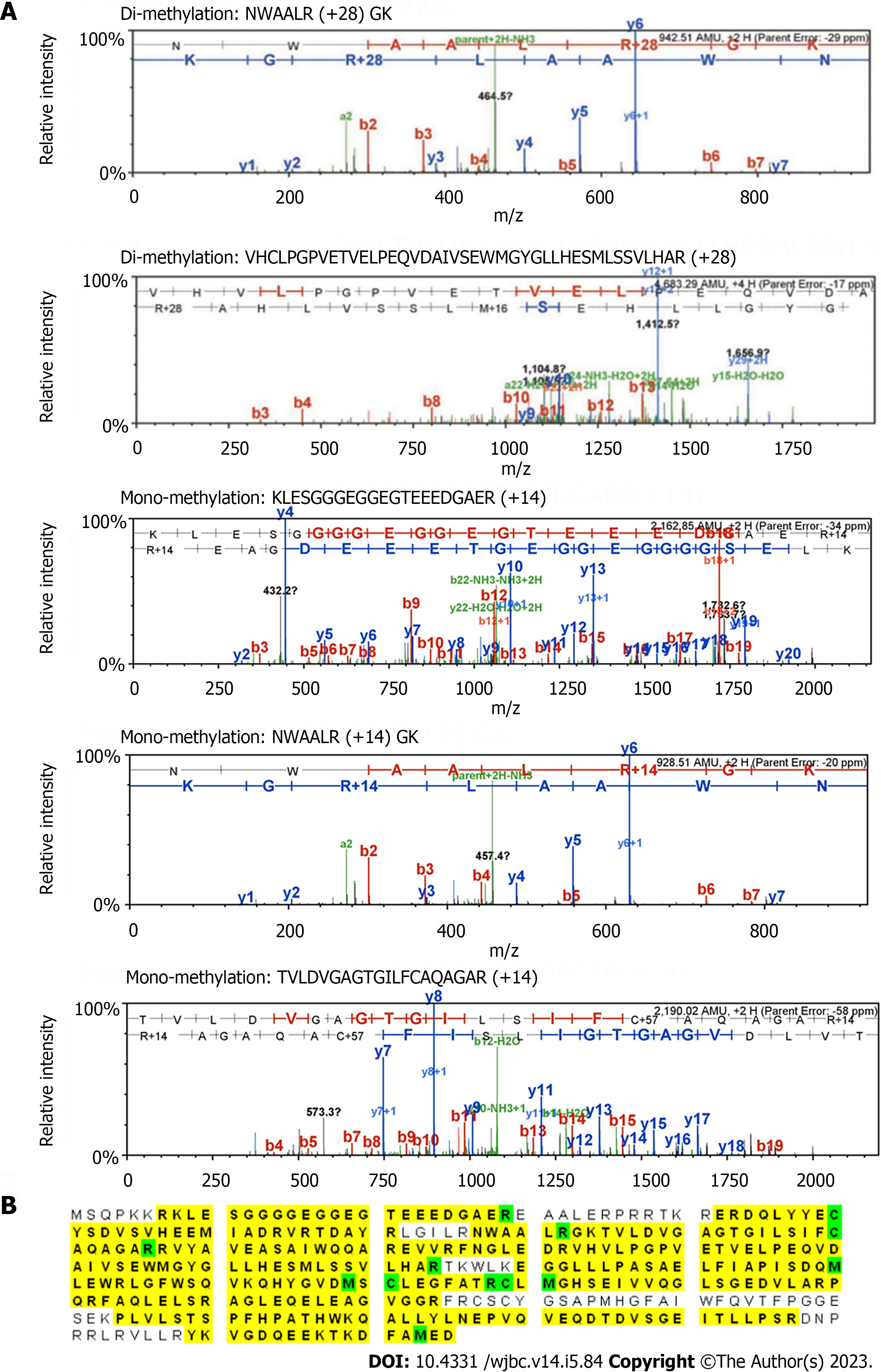
Figure 4 Liquid chromatography with tandem mass spectrometry analysis of the methylation sites on protein arginine methyltransferase 6.
A: 5.5 M protein arginine methyltransferase (PRMT) 6 and 200 M S-adenosyl methionine were incubated with 1 M PRMT1 at 30 ℃ for 3 h in the reaction buffer. The methylated PRMT6 band was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, cut from the gel, washed and vacuum-dried. The sample was sent to UAB proteomics and mass spectrometry facility (Birmingham, AL 35294) for in-gel digestion and liquid chromatography-tandem mass spectrometry (MS/MS) detection. Tandem MS spectra showing the arginine-dimethylated peptides; B: PRMT6 protein sequence coverage (yellow) and the modified residues (green) detected by tandem MS analysis. Green "C" is with carboamidomethylation, and green "M" is with oxidation.
- Citation: Cao MT, Feng Y, Zheng YG. Protein arginine methyltransferase 6 is a novel substrate of protein arginine methyltransferase 1. World J Biol Chem 2023; 14(5): 84-98
- URL: https://www.wjgnet.com/1949-8454/full/v14/i5/84.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v14.i5.84