Copyright
©The Author(s) 2023.
World J Biol Chem. Oct 17, 2023; 14(5): 84-98
Published online Oct 17, 2023. doi: 10.4331/wjbc.v14.i5.84
Published online Oct 17, 2023. doi: 10.4331/wjbc.v14.i5.84
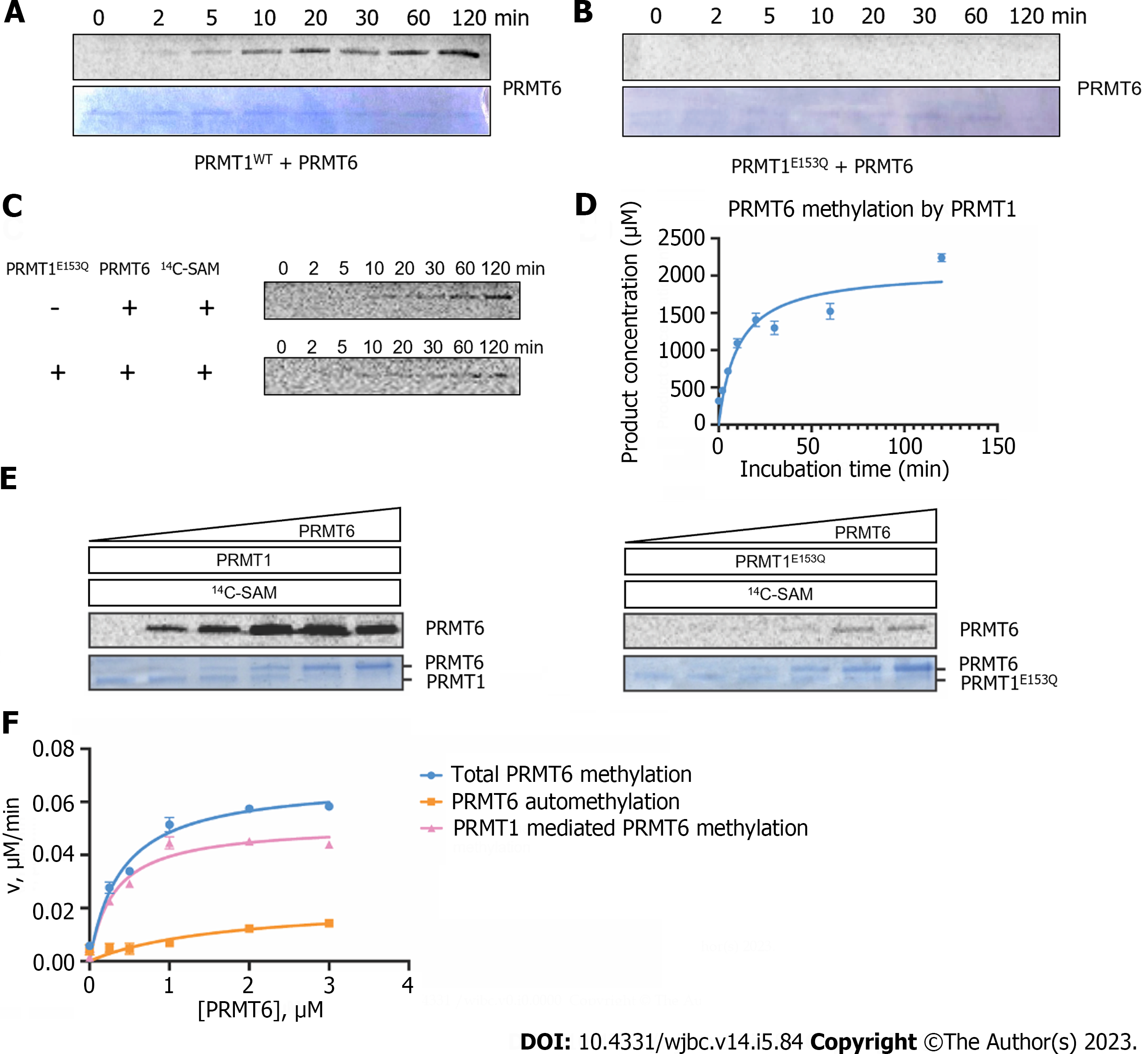
Figure 3 Time-dependent and concentration-dependent protein arginine methyltransferase 6 methylation catalyzed by protein arginine methyltransferase 1.
A and B: Time-dependent radioactive gel methyltransferase assays were carried out at 30 ℃ in an incubation system of 30 µL. 1.5 µM of protein arginine methyltransferase (PRMT) 6 and 10 µM of 14C-S-adenosyl methionine (SAM) were incubated with 0.4 µM of PRMT1WT or PRMT1E153Q, and the reactions were quenched at different time points (0-120 min) by sodium dodecyl sulfate (SDS) loading buffer. The reaction mixture was separated on 12% SDS-polyacrylamide gel electrophoresis (PAGE) and methylated PRMT6 were visualized by Coomassie blue staining and phosphor image scanning, respectively; C: Gel electrophoresis autoradiography assays were performed at 30 ℃ in an incubation system of 30 µL. 4.5 µM PRMT6 was incubated with 10 µM 14C-SAM with or without 0.4 µM of PRMT1E153Q; D: Time course of PRMT6 methylation with PRMT1, the linear part of the curve was used to calculate the steady-state rate; E: Concentration-dependent radioactive gel methyltransferase assay was performed. Different concentration of PRMT6 (0, 0.25, 0.5, 1, 2, 4 M) was incubated with 10 M 14C-SAM in the presence of 0.2 M of PRMT1WT or PRMT1E153Q in the reaction buffer at 30 ℃ for 1h. The reaction mixture was separated on 12% SDS-PAGE and the phosphor image was quantified by ImageJ. Data were normalized according to the reading of 5 M 14C-BSA and the reaction yield was calculated according to the reading of 5 M reaction mixture in liquid scintillation; F: Comparison of the total PRMT6 methylation in the presence of PRMT1, PRMT6 automethylation, and PRMT1-mediated PRMT6 methylation at different concentration of PRMT6. SAM: S-adenosyl methionine; PRMT: Protein arginine methyltransferase.
- Citation: Cao MT, Feng Y, Zheng YG. Protein arginine methyltransferase 6 is a novel substrate of protein arginine methyltransferase 1. World J Biol Chem 2023; 14(5): 84-98
- URL: https://www.wjgnet.com/1949-8454/full/v14/i5/84.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v14.i5.84