Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. Dec 26, 2010; 1(12): 369-376
Published online Dec 26, 2010. doi: 10.4331/wjbc.v1.i12.369
Published online Dec 26, 2010. doi: 10.4331/wjbc.v1.i12.369
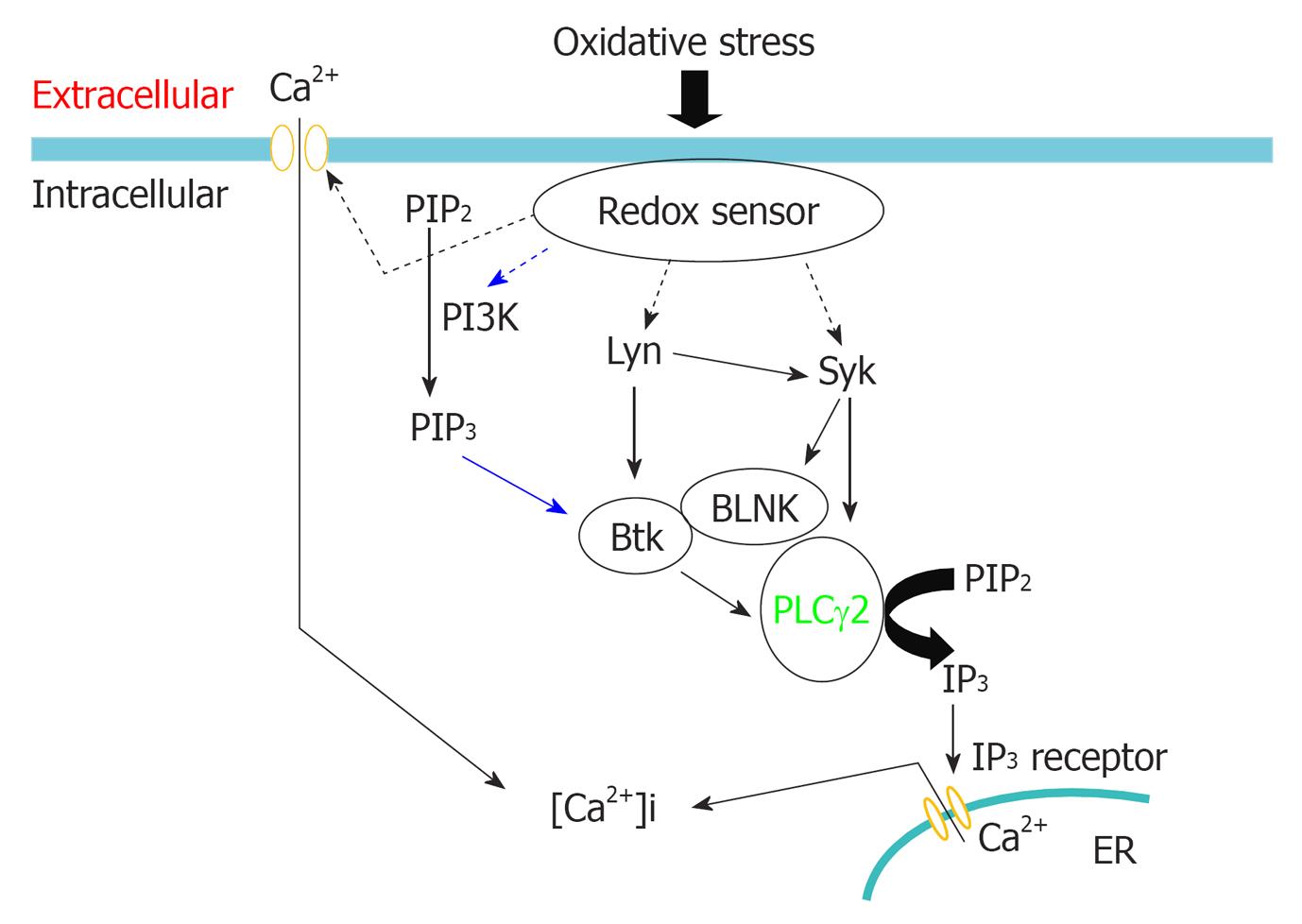
Figure 3 Proposed model for Syk in mediating oxidative stress-induced calcium release.
Phosphorylation of Syk by Lyn and autophosphorylation is required for oxidative stress-induced activation of Syk. How Lyn senses oxidative stress in DT40 cells is currently unknown. Oxidative stress might inhibit tyrosine phosphatase, thereby activating Lyn; Activated Syk phosphorylates phospholipase Cγ2 (PLCγ2) and adaptor protein B cell linker protein (BLNK). Phosphorylated BLNK provides docking sites for PLCγ2 and Btk, enable Btk phosphorylates PLCγ2. Simultaneous phosphorylation of PLCγ2 by Syk and Btk is indispensable for oxidative stress-induced activation of PLCγ2; Activated PLCγ2 cleaves phosphatidylinositol 4,5-biphosphate, releases IP3, triggering Ca2+ release via binding to ryanodine receptor. Oxidative stress also activates PI3K pathway signaling through recruitment of p85 PI3K to the particulate fraction, producing phosphatidylinositol 3,4,5-trisphosphate that targets the activated PLCγ2 to its substrate site for maximal catalytic efficiency.
- Citation: Qin S. Suofu Qin’s work on studies of cell survival signaling in cancer and epithelial cells. World J Biol Chem 2010; 1(12): 369-376
- URL: https://www.wjgnet.com/1949-8454/full/v1/i12/369.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i12.369