Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. Dec 26, 2010; 1(12): 369-376
Published online Dec 26, 2010. doi: 10.4331/wjbc.v1.i12.369
Published online Dec 26, 2010. doi: 10.4331/wjbc.v1.i12.369
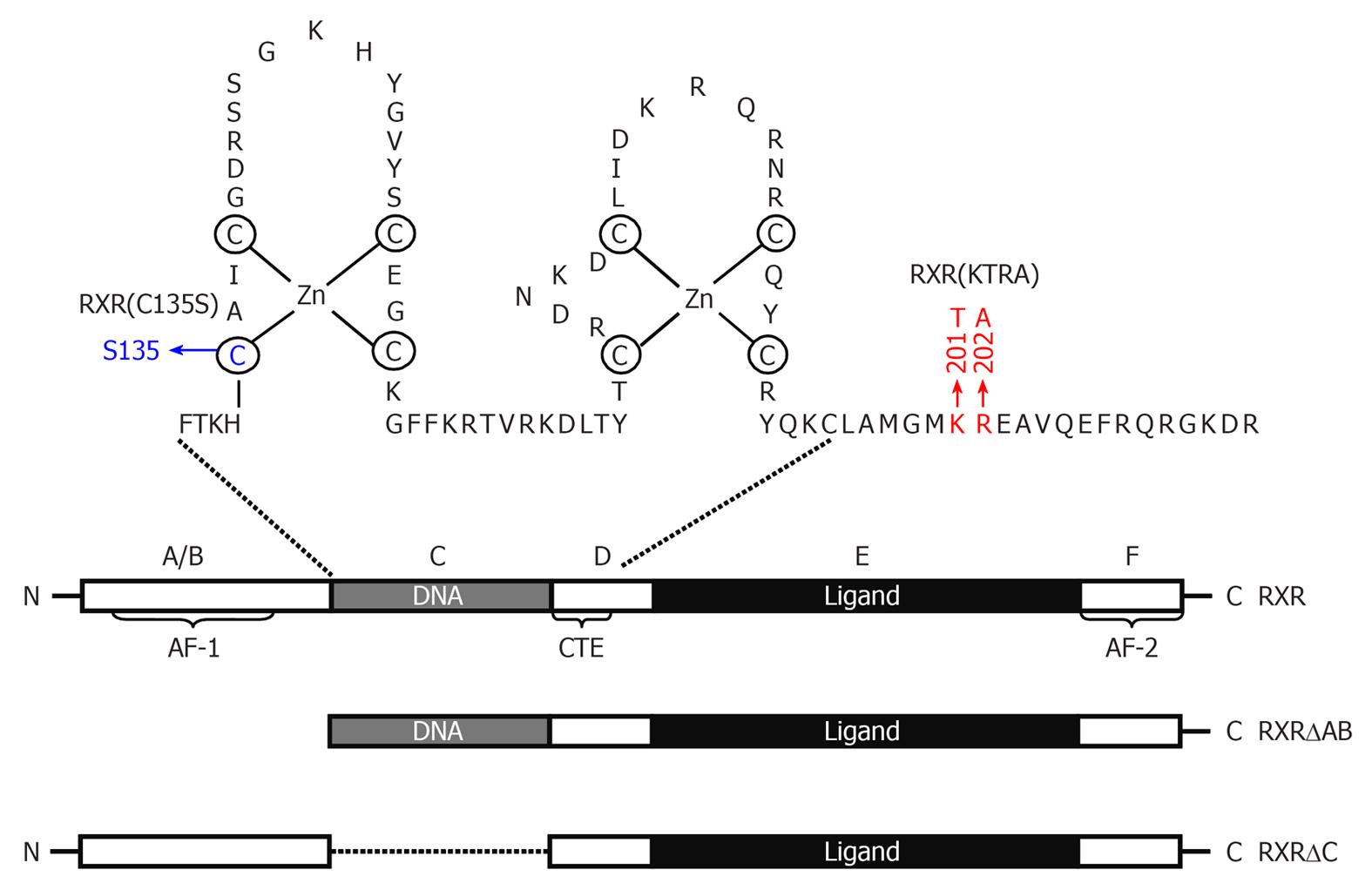
Figure 2 Schematic representation of Retinoid X receptor α protein and its deletion mutants.
Retinoid X receptor (RXR)α consists of a variable N-terminal domain (A/B domain) containing activation function 1, a highly conserved DNA-binding domain (DBD), also named as C domain, a nonconserved hinge (D domain), a moderately conserved ligand-binding domain (E domain), and a ligand-dependent transactivation F domain (AF-2). The DBD consists of two cysteine-rich zinc-finger motifs through which nuclear receptors bind to a specific DNA sequence and a carboxy-terminal extension (CTE) that exists only in RXRs and RXR-binding partners. Point mutation of Cys135 to Ser in first zinc-finger disables the capability of RXR to bind DNA. KTRA mutations in the CTE disrupts RXRα homodimerization but does not affect RXRα heterodimerization. Ligand-binding domain contains a ligand-binding pocket for binding of small, lipophilic molecules, a cofactor binding surface, and a dimerization surface.
- Citation: Qin S. Suofu Qin’s work on studies of cell survival signaling in cancer and epithelial cells. World J Biol Chem 2010; 1(12): 369-376
- URL: https://www.wjgnet.com/1949-8454/full/v1/i12/369.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i12.369