Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. Dec 26, 2010; 1(12): 353-361
Published online Dec 26, 2010. doi: 10.4331/wjbc.v1.i12.353
Published online Dec 26, 2010. doi: 10.4331/wjbc.v1.i12.353
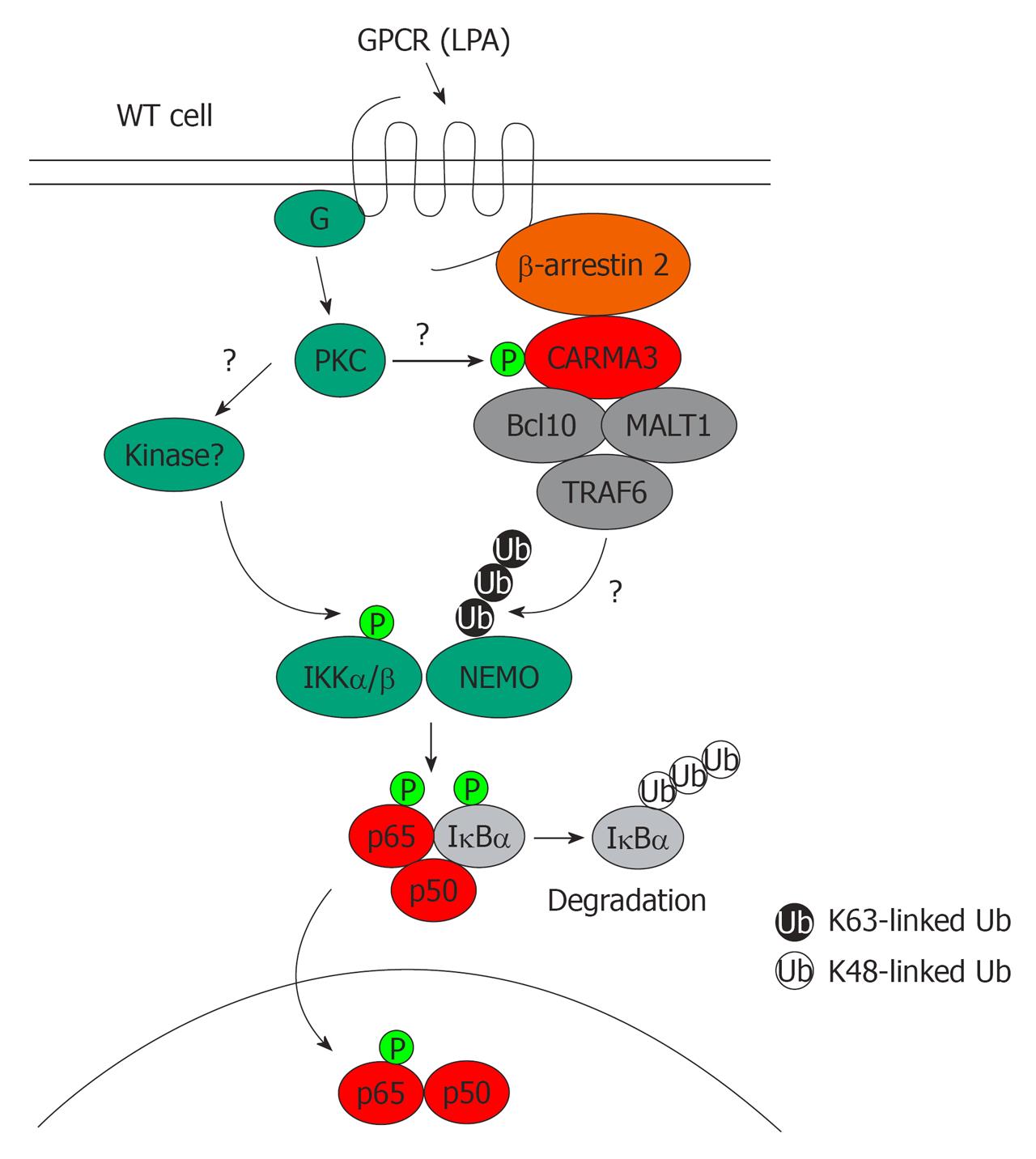
Figure 2 Working model of CARD recruited membrane associated protein 3-dependent nuclear factor-κB activation in the G protein-coupled receptor (lysophosphatidic acid) signaling pathways.
G protein-coupled receptor (GPCR) [lysophosphatidic acid (LPA)]-induced nuclear factor (NF)-κB activation involves the recruitment of CARD recruited membrane associated protein 3 (CARMA3) to the receptor by β-arrestin 2, which leads to formation of the CARMA3/Bcl10/MALT1/TRAF6 complex, which results in polyubiquitination of the IκB kinase (IKK) complex. A CARMA3-independent, PKC-dependent signal induces phosphorylation of the IKK complex by an unknown kinase in the presence of GPCR. After IKK is both polyubiquitinated [NF-κB essential modulator (NEMO)] and phosphorylated (IKK), it is activated, which leads to NF-κB activation. In the absence of CARMA3, GPCR (LPA)-induced polyubiquitination of the IKK complex is defective, which results in defects in IKK and NF-κB activation. WT: Wild-type; Ub: Ubiquitin.
- Citation: Sun J. CARMA3: A novel scaffold protein in regulation of NF-κB activation and diseases. World J Biol Chem 2010; 1(12): 353-361
- URL: https://www.wjgnet.com/1949-8454/full/v1/i12/353.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i12.353