Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. Oct 26, 2010; 1(10): 313-320
Published online Oct 26, 2010. doi: 10.4331/wjbc.v1.i10.313
Published online Oct 26, 2010. doi: 10.4331/wjbc.v1.i10.313
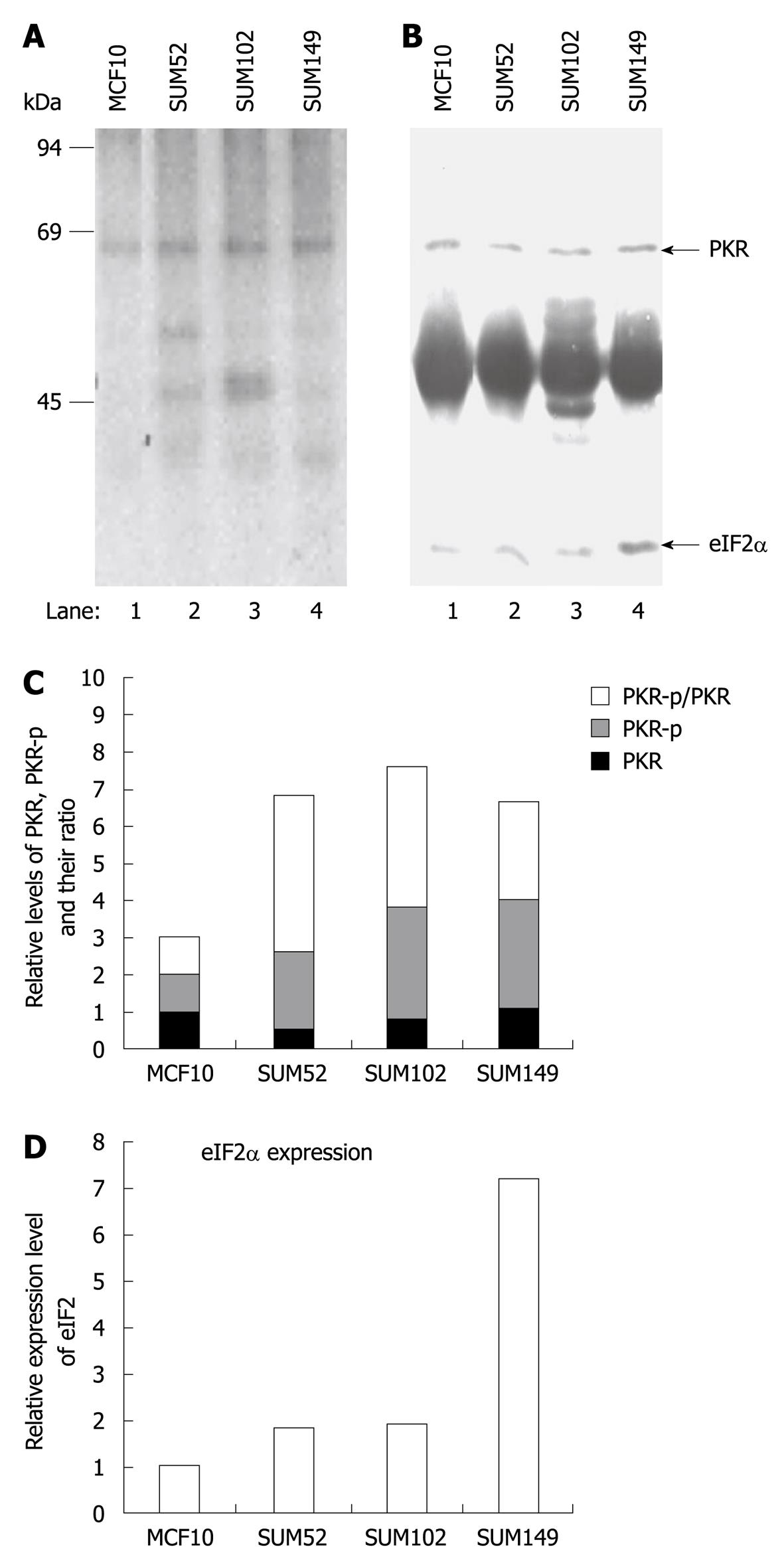
Figure 5 In vivo protein kinase autophosphorylation and eukaryotic translation initiation factor 2 phosphorylation in normal breast and breast cancer cells.
One established breast cell line (MCF-10, lane 1) and three primary breast cancer cell lines (lanes 2-4) were labeled with [32P] phosphoric acid, and cell extracts were prepared by lysis in NP-40 lysis buffer. Protein kinase (PKR) and eukaryotic translation initiation factor 2 (eIF2α) were immunoprecipitated with corresponding antibodies, resolved by SDS-PAGE, and electroblotted to nitrocellulose. A: Incorporation of 32PO4 into PKR and eIF2α was measured by autoradiography; B: Protein levels of PKR and eIF2α on the same membrane were determined by Western blotting analysis; C and D: Semi-quantitative analysis of the levels of phosphorylated PKR, total PKR and eIF2α using ImageJ (v 1.42k, NIH). The levels of the proteins in the cancer cell lines were normalized with the level of the same proteins in MCF10 cells.
- Citation: Wu S. Localization and function of a eukaryotic-initiation-factor-2-associated 67-kDa glycoprotein. World J Biol Chem 2010; 1(10): 313-320
- URL: https://www.wjgnet.com/1949-8454/full/v1/i10/313.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i10.313