Published online Mar 27, 2012. doi: 10.4240/wjgs.v4.i3.45
Revised: November 4, 2011
Accepted: November 12, 2011
Published online: March 27, 2012
To investigate the progress in evidence-based surgical treatment of non-metastatic gastric cancer, we reviewed the last ten years’ literature. The data used in this review were identified by searches made on MEDLINE, Current Contents, PubMed, and other references taken from relevant original articles (on prospective and retrospective studies) concerning gastric cancer surgery. Only papers published in English between January 1999 and December 2009 were selected. Data from ongoing studies were obtained in December 2009, from the trials registry of the United States National Institutes of Health (http://www.clinicaltrial.gov). The citations list was presented according to evidence based relevance (i.e., randomized controlled trials, prospective studies, retrospective series). In the last ten years, many challenges have been faced relating to the extension of gastric resection and nodal dissection as well as surgical timing, but we found only limited evidence, regardless of latitude of study. The ongoing phase-III trials may provide answers that will be valid for the coming decades, and which may bring definitive answers for the currently unresolved questions.
- Citation: Rausei S, Dionigi G, Rovera F, Boni L, Valerii C, Giavarini L, Frattini F, Dionigi R. A decade in gastric cancer curative surgery: Evidence of progress (1999-2009). World J Gastrointest Surg 2012; 4(3): 45-54
- URL: https://www.wjgnet.com/1948-9366/full/v4/i3/45.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v4.i3.45
In the current era of evidence-based medicine, the year 1999 seemed to provide several definitive answers regarding the management and, more specifically, the surgical treatment of gastric cancer. Since then, the extension of gastric resection and nodal dissection as well as surgical timing have achieved clear and convincing definitions due to well-designed, randomized, controlled phase-III trials[1-4].
In March 1999, almost simultaneously, Cuschieri et al[2] and Bonenkamp et al[3] discouraged the routine use of D2 lymph node dissection in patients with gastric cancer because of high morbidity risks without any clear survival benefit. Five months later, Bozzetti et al[1] stated that in patients with distal gastric cancer, subtotal gastrectomy can achieve the same outcome as total gastrectomy, with the only difference being that the former procedure provides a better quality of life.
Taken together, these conclusions appeared to decrease the relevance of surgical exeresis in gastric cancer treatment. The neoadjuvant therapy approach, suggested by several phase-II trials[5-9], indicated a in the same direction, resulting in further questioning of surgical timing.
However, in April 1999, Songun et al[4] were obliged to stifle their enthusiasm for the supposed efficacy of pre-operative chemotherapy due to an unacceptable tumor progression rate, allowing some surgeons the (short-lived) belief that they still held exclusive rights over the cure.
Over the last decade, many trials concerning gastric cancer surgery have been performed, and many of the earlier conclusions have either been changed or reversed.
More than a decade after the publication of the above-cited “revolutionary” randomized studies, we sought to review the specific literature and to report the progress in evidence-based surgical treatment of non-metastatic gastric cancer.
During the decade 1999-2009, many challenges were addressed, but we found only a limited amount of evidence, regardless of the latitude of our searches.
The data used in this review were identified by searches made on MEDLINE, Current Contents, PubMed, and other references taken from relevant original articles (on prospective and retrospective studies) concerning gastric cancer surgery.
Only papers published in English between January 1999 and December 2009 were selected. Data from ongoing studies were obtained in December 2009, from the trials registry of the United States National Institutes of Health (http://www.clinicaltrial.gov).
The authors attributed an evidence category to the retrieved studies according to the well-known classification (Table 1)[10,11]. The citations list was presented according to evidence based relevance (i.e., randomized controlled trials, prospective studies, retrospective series).
| Level of evidence | Type of trial | Criteria for classification | Grade of recommendation |
| I | Large randomized trials with clear-cut results (and low error risk) | Sample size calculation provided and fulfilled; study endpoint provided | A |
| II | Small randomized trials with uncertain results (and moderate to high error risk) | Matched analysis, sample size calculation not given or not fulfilled; study endpoints not provided; convincing comparative studies | B |
| III | Nonrandomized, contemporaneous controls | Noncomparative, prospective | C |
| IV | Nonrandomized, historical controls | Retrospective analysis, cohort studies | - |
| V | V No control, case series only; experts opinions | Small series, review articles | - |
Starting from the publication of the Bozzetti et al trial, and subsequently confirmed by a number of retrospective studies[12,13], the aim of achieving adequate gastric resection through 5-cm negative margins contributed to the worldwide spread of the concept of limited gastrectomy, regardless of the histological type of tumor.
However, although evidence on subtotal gastrectomy for distal tumors has now reached a relevant level (evidence level: I A; Table 2)[1], proximal gastrectomy for cancer of the upper third still remains controversial.
| Topic | Recommendation | Ref. |
| Traditional controversies | ||
| Extension of resection | Subtotal gastrectomy with 5 cm negative margins is sufficient for the curative treatment of distal tumors | [1] |
| Prophylactic splenectomy is not necessary for cardia tumors either | [25-27] | |
| Extension of lymphadenectomy | D2 nodal dissection with spleno-pancreasectomy does not provide any survival benefit and increases post-operative morbidity and mortality rates | [2-3] |
| Pancreas-preserving D2 nodal dissection increases survival rates without any significant post-operative morbidity and mortality | [44] | |
| Para-aortic nodal dissection (in addition to D2 lymphadenectomy) does not improve the survival rate in curable diasease | [45] | |
| Surgery in multimodal strategy | ||
| Pre-operative chemotherapy | Pre-operative chemotherapy is associated to an increase in survival rates | [121,122] |
Despite the promising results of some small eastern prospective studies[14,15], several retrospective analyses[16-18] reported a lack convincing long-term results for this procedure whilst demonstrating a high frequency of complications (anastomotic stenosis and reflux esophagitis) and cancer recurrences. This has significantly affected diffusion of this procedure (evidence level: II B).
Driven by the additional morbidity risks and the poor nutritional status associated with extension of gastric resection[19], several authors suggested either a wedge resection or a pylorus-preserving sleeve gastrectomy, at least for the treatment of early disease[20-24]. However, there still is a lack of data to support the adoption of these solutions (evidence level: II B).
Similarly, the reports published over the last decade suggest that we still have not achieved a definition of surgical overtreatment. Although good evidence has discouraged prophylactic splenectomy in cardiac tumors[25-27] (evidence level: I A; Table 2) and although we are currently awaiting the results of the JCOG0110 trial (Table 3)[28], retrospective analysis by many authors has led to their recommending the aggressive combined resection of adjacent organs for patients with T4 carcinoma[29-32]. However, the possibility of obtaining a curative resection in the presence of a supposed T4 cancer is not always predictable. In fact, although histopathological examination reveals that multi-organ resections are often performed for pT3 tumors, a relatively small proportion of tumors are actually invading adjacent organs[33]. The likelihood of non-curative exeresis for supposed T4 cancers increases to approximately 70%[31]. Therefore, accurate selection of patients for multi-organ resection based on lymph nodal status has been proposed[31,32], similar to the recommendation of the completion of exeresis after a gastrectomy with positive surgical margins[34-37]. However, the evidence does not support any of these extreme procedures (evidence level: IV C).
| Topic | Title of trial | Institution | Estimated enrollment | Study start year | Registration identifier |
| Traditional controversies | |||||
| Extension of resection | GCSSG-SPNX: Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma: JCOG0110 | Japan Clinical Oncology Group-Japan | 500 | 2002 | NCT00112099[28] |
| Extension of lymphadenectomy | A Comparison Between D1 and D2 Lymphadenectomy in Gastric Cancer: A Prospective Randomized Controlled Trial | Tata Memorial Hospital-India | 600 | 2007 | NCT00447746[46] |
| Minimally invasive approach | |||||
| Laparoscopic resection | Prospective Randomized Trial of Laparoscopy-Assisted Distal Gastrectomy (LADG) Versus Open Distal Gastrectomy (ODG) in Patients With Early Gastric Cancer (EGC) | National Cancer Center-Korea | 164 | 2003 | NCT00546468[100] |
| Multi-Institutional Prospective Randomized Trial on the Assessment of Laparoscopic Surgery for Gastric Cancer | National Cancer Center-Korea | 1400 | 2006 | NCT00452751[101] | |
| Surgery in multimodal strategy | |||||
| Pre-operative chemotherapy | Randomized Phase III Trial of Surgery Plus Neoadjuvant TS-1 and Cisplatin Compared With Surgery Alone for Type 4 and Large Type 3 Gastric Cancer: Japan Clinical Oncology Group Study (JCOG 0501) | Japan Clinical Oncology Group-Japan | 316 | 2005 | NCT00252161[123] |
| A Multicenter Randomized Phase III Trial of Neo-Adjuvant Chemotherapy Followed by Surgery and Chemotherapy or by Surgery and Chemoradiotherapy in Resectable Gastric Cancer (CRITICS Study) | Dutch Colorectal Cancer Group-Netherlands | 788 | 2006 | NCT00407186[125] | |
| A Randomized Controlled Phase II/III Trial of Peri-Operative Chemotherapy With or Without Bevacizumab in Operable Adenocarcinoma of the Stomach and Gastro Oesophageal Junction | Medical Research Council-United Kingdom | 1100 | 2007 | NCT00450203[124] | |
While Bozzetti et al[1] opened the road towards limited surgery for gastric cancer treatment, Cuschieri et al[2] (MRC trial) and Bonenkamp et al[3] (Dutch trial) seem to have stopped the use of extended lymphadenectomy that had began in the 1970s in eastern countries. These randomized controlled trials (RCTs) revealed no survival benefit for D2 nodal dissection with spleno-pancreatectomy and confirmed an increase in morbidity related to this procedure, thus leading many surgeons to abandon this strategy (evidence level: I A; Table 2).
However, the rereading of data contained in the Dutch study after a long-term follow-up revealed an improved survival trend for selected patients who had D2 dissection[38]. Moreover, a further reanalysis of the trial performed by Bonenkamp and colleagues, which excluded both distal pancreatectomy and splenectomy cases (as these are a significant factor in post-operative morbidity), found a survival benefit for patients who underwent a D2 resection[39]. This finding was later confirmed by two small phase-II prospective studies carried out by Italian and UK groups[40,41], both of which concluded that modified D2 gastrectomy (without routine spleno-pancreatectomy) could improve survival for gastric cancer patients, without any significant increase in morbidity or mortality compared to D1 gastrectomy. Therefore, with the new-born concept of the minimum number of removed lymph nodes for staging in the 5th edition of the TNM (UICC, 1997)[42], and just five years after the appearance of the results of both the Dutch and MRC trials, the conclusions about lymphadenectomy were evolved rapidly.
Recommendations radically changed in 2006, when data from the first RCT concerning the efficacy of modified D2 lymphadenectomy (named D3 by the authors, according to the first edition of the Japanese classification of gastric cancer[43]) were published[44]. Wu et al confirmed, in a well-designed study of over 200 gastric cancer patients, that compared with D1 nodal dissection, D2 dissection offers a survival benefit only if performed by well-trained, experienced surgeons (evidence level: I A; Table 2). The last conclusion was derived from the knowledge that an extended modified nodal dissection could be associated with an increase in morbidity (with no mortality) rates that was far from the disturbing rates recorded in previous trials.
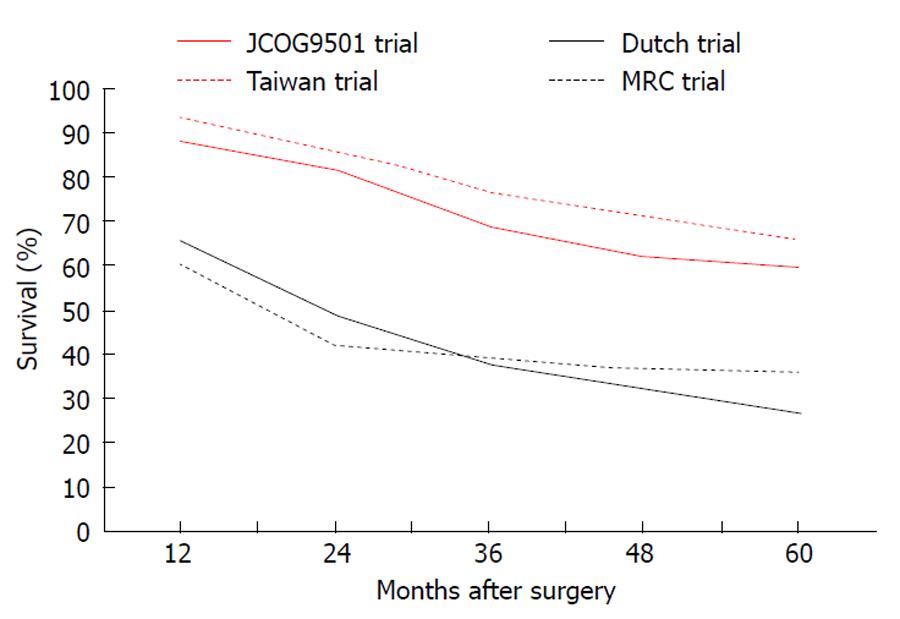
Similar concerns have also been expressed by the authors of the JCOG9501 trial relating to the comparison between D2 lymphadenectomy “alone” or associated with para-aortic nodal dissection[45]. This study presented clear reassurance on the efficacy of modified D2 gastrectomy (Figure 1), also confirming the trend towards higher morbidity and excluding any survival benefit for more extended lymphadenectomy.
Interestingly, the duration of this controversy induced authors to design even larger trials to confirm a well-known answer (e.g., the NCT00447746 trial from Tata Memorial Centre Mumbai, India) (Table 3)[46].
Because the recent philosophy “from maximum tolerable, to minimum effective therapy” has also gained a footing in gastric cancer management, the (industrial) push towards the minimally invasive approach seems to be unstoppable.
Even though there is no strong evidence concerning the lowest extension limit of gastric resection, we will probably obtain more evidence in the near future from new technological approaches rather than from more conventional methods (i.e., wedge resection or sleeve gastrectomy pylorus-preserving). This will only happen if the slow and rigorous approach of RCTs is able to keep pace with technological evolution.
Meanwhile, we would like stress that the current final goal is the most “effective” therapy rather than necessarily the “minimum” one.
Over the last decade, endoscopy has been considered to be an adequate therapeutic option for the cure of early gastric cancer. It has been both included into eastern guidelines for gastric cancer treatment[47] and cited in the clinical practice guidelines of the National Comprehensive Cancer Network in the United States[48].
Currently, the literature (predominantly coming from the Asian continent) relating to therapeutic endoscopy [endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD)] is very broad[49-69] and already includes some review articles aimed at summarizing a number of different techniques, as well as comforting results for he two modalities (EMR, ESR) in terms of resection rates, morbidity and survival[70,71].
However, the evidence supporting the use of these modalities is limited, especially considering the complete absence of randomized controlled studies (many retrospective and few prospective series; evidence level: III C).
For this reason, although the scientific community still anticipates a satisfactory survival comparison between EMR (presented in the 1970s) and conventional surgery, several recent reports have provided (from the late 1990s) a similarly positive recommendation for ESD (even if not supported by large samples and long-term outcome data).
The efficacy of removing more advanced lesions without any size limitation is undoubtedly superior with ESD rather than with conventional EMR, even though this technique carries several disadvantages, such as a longer procedure time together with a higher post-operative bleeding and perforation rate.
Therefore, ESD has introduced great changes in the endoscopic treatment of early cancers and extension of its indications has been suggested: from differentiated lesions up to 20 mm in diameter without ulceration in the EMR era, to 30-mm ulcerative or submucosal (less than 500 μm) differentiated lesions in the ESD era[72].
One remarkable issue concerns the recruitment criteria for endoscopic treatment, which were designed on the basis of fundamental prognostic considerations, stating the likelihood of the presence of metastatic lymph nodes calculated by observational studies[73-78]. As shown above, these criteria have been extended[72,79] and, although there are some questions about their suitability[80], several authors encourage their further extension[81-83]. Moreover, as in primary treatment, in the case of incomplete resection after EMR or ESD, an additional endoscopic procedure (within the limits of the histopathologically verified extended criteria) has been suggested[84-86].
Recently, the phase-II JCOG 0607 study set out to verify the safety of the extended criteria, which appear to be already included in the Japanese guidelines[87].
The laparoscopic approach to gastric cancer surgery was adopted in eastern countries from the beginning of the 1990s and presented encouraging results in terms of both feasibility and safety.
However, laparoscopy for gastric cancer treatment still has not reached the same evidence strength as laparoscopic colorectal cancer surgery. The well-known technical difficulties and oncological concerns, delayed the widespread use of this approach in its initial phase.
A considerable number of series and comparative studies have focused on laparoscopic resection for gastric cancer, especially in early and distal disease. Results have suggested that totally laparoscopic distal gastrectomy (as well as laparoscopy-assisted procedure, hand-assisted laparoscopic procedure or, more recently, robot-assisted laparoscopic procedure) is, compared to open gastrectomy, associated with a quicker return of gastrointestinal function, faster discharge from the hospital as well as comparable complications and recurrence rates[88-94]. These findings have resulted in the inclusion of this modality of treatment in the Japanese guidelines for gastric cancer since 2001[95].
However, to date, the English literature does not present any large randomized studies with clear-cut results regarding this issue. In fact, the only four RCTs containing survival data from long-term follow-up[96-99] recruited less than one hundred patients (pooled: 82; the trial by Huscher et al also included advanced disease). It is because of these small numbers that the evidence supporting laparoscopic treatment is so weak (evidence level: II B), and, as a consequence, widespread adoption of the procedure cannot be recommended.
Nonetheless, this problem should be solved by ongoing phase-III trials (both Korean), which aim at recruiting a ten-fold higher number of patients than previous RCTs[100,101] (Table 3).
As suggested by some recent reports, even once the safety and efficacy of laparoscopic distal gastrectomy have been demonstrated, there will remain problems regarding evidence for laparoscopic resection[102-107]. First, the efficacy of laparoscopic gastrectomy in proximal early disease (also laparoscopic gastrectomy and extended lymphadenectomy for advanced tumors) and the territorial line between the indications for either minimally invasive (laparoscopy) or organ conservative surgery (endoscopy) will need to be demonstrated. Second, the possibility of generalizing laparoscopic (as well as endoscopic) results should be verified because all pioneering studies are likely to favor the new method, as their authors are experts in the field.
Although sentinel node navigation aims at surgical cancer staging and not surgical cure, we will examine this “staging modality,” as it was introduced with the purpose of confirming the oncological clearance of the minimally invasive approach for primary tumors (EMR/ESD, laparoscopic or open limited resection). For patients who do not have any clinical demonstration of metastatic lymph nodes, some surgeons have suggested that a sentinel node procedure might avoid the complications resulting from overtreatment by an extended lymphadenectomy[24,108-112].
However, the centrifugal nature of the lymphatic flow from the stomach has resulted in skepticism towards the efficacy of this technique associated to gastrectomy, and, to date, no series have demonstrated a false negative rate which is acceptably close zero.
The available literature reports a sensitivity of 72%-93%, a specificity of 75% and an accuracy of 74%-100%, but a negative predictive value of 50%[111-115]. The reasons for these wide ranges will be indicated hereafter.
The first issue resides in the absence of a standardized approach for the identification of the sentinel node (cancer-injected endoscopically or intra-operatively, in either the sub-mucosa or subserosa, using a dye or radioactive tracer; sentinel node identified by direct visualization or by hand-held gamma probe). The second problem arises from the possible presence of lymphatic invasion of early tumors included in the analyses. In such cases there might be a false negative sentinel node because the lymphatic vessels have been completely obstructed by the cancer.
Hence, the usefulness of sentinel node navigation in influencing the patient’s care has not yet been established, and is probably still far from being established (evidence level: III C). Additionally, this issue has never been taken into consideration in a randomized trial setting.
The rationale of the first RCT, published by a Dutch group in 1999, concerning pre-operative chemotherapy was driven by a number of considerations, such as the demonstrated higher morbidity/mortality rates and lack of any survival benefits after extended lymphadenectomy and the poor efficacy of adjuvant regimens in locally advanced diseases[4]. Hence, this trial attempted, albeit unsuccessfully, to integrate the unsatisfactory surgical results with a pre-operative tumor downstaging therapy aimed at reducing the risk of disease recurrence after surgery.
At the present time, the rationale of neoadjuvant chemotherapy is based on different premises, but still has the same purpose. Although extended lymphadenectomy has added efficacy to gastric cancer surgery[44-45] and effective post-operative schedules have been introduced[116,117], worldwide mortality still remains unacceptable for patients with resectable T3 and T4 tumors or other types of resectable gastric cancer involving the lymph nodes[45,116,118].
Therefore, many efforts, using a multimodal strategy and a pre-operative approach, have been performed[5-9,119,120]. In 2006, the MAGIC and the FFCD 9703 trials[121,122] started to provide concrete evidence for neoadjuvant chemotherapy, definitively introducing the concept of “delayed surgery” in gastric cancer treatment.
Today, gastric cancer surgeons should be able to postpone surgical exeresis in the case of a patient with a resectable locally advanced tumor. Indeed, it is widely recognized that this type of patient might experience an effective survival benefit in response to a neoadjuvant treatment comprising pre-operative chemotherapy with epirubicin, cisplatin and 5-FU[121,122] (evidence level: I A; Table 2). However, surgeons should also be aware of the concerns arising from pre-operative treatment. In fact, the resection delay (within three to six weeks after completion of the third three-week cycle of chemotherapy) does not exclude patients from the benefits of a potentially curative postponed resection and does not worsen surgical outcomes, even though some patients might be affected by tumor progression.
For these reasons, this issue undoubtedly needs further investigation not only to determine the size of the problem, but also to reduce its incidence, to optimize pre-treatment staging and, finally, to improve the biomolecular characterization of each cancer. The primary aim is the selection of patients who will benefit from pre-operative treatment. Consequently, the topic of the neoadjuvant approach remains “hot” and is the focus of several ongoing phase-III RCTs (Table 3): the JCOG trial 0501 (Japan Clinical Oncology Group Study 0501 trial-completed, but still awaiting publication of results)[123]; the MAGIC B trial (United Kingdom National Cancer Research Institute ST03 trial -in recruitment phase)[124]; and the CRITICS study (Dutch Colorectal Cancer Group trial -in recruitment phase)[125] (Table 3).
Importantly, the latter study is investigating the efficacy, within the adjuvant setting, of the association of radiotherapy with chemotherapy, simultaneously seeking to confirm the consolidated evidence for pre-operative chemotherapy through the study design. The role of radiotherapy in the neoadjuvant setting has also been tested, and phase-II trials are currently giving encouraging results[126,127] (evidence level: III C). Thus, the role of radiotherapy in the neoadjuvant setting is worth exploring further in clinical phase-III RCTs.
However, we do not want to dwell on the different therapeutic modalities for achieving (pre-operatively, intra-operatively and post-operatively) the curative effect of surgery. Instead, we want to underline the possibility of modifying the timing of surgery by safe postponement of gastrectomy.
In the era of evidence-based medicine, RCTs are widely accepted as the gold standard tool for comparison of different therapeutic modalities. The random allocation of a large sample of patients should avoid selection biases and generate an equal distribution of prognostic factors among the study groups.
The reliability of trial results is further enhanced by application of methods such as independent investigators, blinding techniques and therapy standardization.
Although the basic principles of trial methodology may be considered to be valid for surgical research as well, surgical situations that would require a RCT approach often present several factors [e.g., patients’ preferences (selection bias), surgeons’ preferences and lack of blinding or placebo (treatment bias)] that may complicate or, in some extreme cases, even prevent the conduction of the analysis.
Therefore, surgical research typically comprises either rigorously performed trials that have a great impact on clinical practice or poorly performed trials in which the validity of the result is questionable.
In the present work we referred, on the basis of these premises, to the modified Sackett’s classification of evidence levels[10,11] (Table 1); this only assigns great credibility to large, well-designed RCTs with clear-cut results, avoiding all possible considerations of the complex and not always accurate statistical processing of meta-analyses.
Nonetheless, we must underline some issues that are of crucial importance for understanding the real progress of evidence levels in gastric cancer surgery.
Firstly, over the last decade, rapid changes in technology and their consequent industrial push might have led to a biased approach in RCTs.
Secondly, the design of surgical trials aimed at detecting even minimal differences (and thus requiring large sample sizes) has not only hindered performance but has also limited the possibility of generalization of outcomes.
Finally, the generalization of treatment findings emerging from trials should be discouraged for non-experts. The modern management of gastric cancer could actually require risky surgical solutions as well as a multimodal approach. Better surgical outcomes and reasonable survival times can be achieved in selected high-volume institutions with sufficiently strong experience in gastric cancer therapy.
Meanwhile, the ongoing phase-III trials may provide definitive answers that will hopefully be valid for the coming decades, and may bring definitive answers for the currently unresolved questions.
Peer reviewer: Mehmet Fatih Can, Assistant Professor, Gulhane School of Medicine, Department of Surgery, Etlik 06018, Ankara, Turkey
S- Editor Wang JL L- Editor Hughes D E- Editor Zhang DN
| 1. | Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg. 1999;230:170-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 260] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522-1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 994] [Cited by in F6Publishing: 982] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 3. | Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1158] [Cited by in F6Publishing: 1220] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 4. | Songun I, Keizer HJ, Hermans J, Klementschitsch P, de Vries JE, Wils JA, van der Bijl J, van Krieken JH, van de Velde CJ. Chemotherapy for operable gastric cancer: results of the Dutch randomised FAMTX trial. The Dutch Gastric Cancer Group (DGCG). Eur J Cancer. 1999;35:558-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Leichman L, Silberman H, Leichman CG, Spears CP, Ray M, Muggia FM, Kiyabu M, Radin R, Laine L, Stain S. Preoperative systemic chemotherapy followed by adjuvant postoperative intraperitoneal therapy for gastric cancer: a University of Southern California pilot program. J Clin Oncol. 1992;10:1933-1942. [PubMed] [Cited in This Article: ] |
| 6. | Ajani JA, Mayer RJ, Ota DM, Steele GD, Evans D, Roh M, Sugarbaker DJ, Dumas P, Gray C, Vena DA. Preoperative and postoperative combination chemotherapy for potentially resectable gastric carcinoma. J Natl Cancer Inst. 1993;85:1839-1844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 98] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Rougier P, Lasser P, Ducreux M, Mahjoubi M, Bognel C, Elias D. Preoperative chemotherapy of locally advanced gastric cancer. Ann Oncol. 1994;5 Suppl 3:59-68. [PubMed] [Cited in This Article: ] |
| 8. | Kelsen D, Karpeh M, Schwartz G, Gerdes H, Lightdale C, Botet J, Lauers G, Klimstra D, Huang Y, Saltz L. Neoadjuvant therapy of high-risk gastric cancer: a phase II trial of preoperative FAMTX and postoperative intraperitoneal fluorouracil-cisplatin plus intravenous fluorouracil. J Clin Oncol. 1996;14:1818-1828. [PubMed] [Cited in This Article: ] |
| 9. | Crookes P, Leichman CG, Leichman L, Tan M, Laine L, Stain S, Baranda J, Casagrande Y, Groshen S, Silberman H. Systemic chemotherapy for gastric carcinoma followed by postoperative intraperitoneal therapy: a final report. Cancer. 1997;79:1767-1775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 10. | Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1989;95:2S-4S. [PubMed] [Cited in This Article: ] |
| 11. | Heinrich S, Schäfer M, Rousson V, Clavien PA. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg. 2006;243:154-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 12. | De Manzoni G, Verlato G, Roviello F, Di Leo A, Marrelli D, Morgagni P, Pasini F, Saragoni L, Tomezzoli A. Subtotal versus total gastrectomy for T3 adenocarcinoma of the antrum. Gastric Cancer. 2003;6:237-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Gockel I, Pietzka S, Gönner U, Hommel G, Junginger T. Subtotal or total gastrectomy for gastric cancer: impact of the surgical procedure on morbidity and prognosis--analysis of a 10-year experience. Langenbecks Arch Surg. 2005;390:148-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Katai H, Sano T, Fukagawa T, Shinohara H, Sasako M. Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg. 2003;90:850-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Yoo CH, Sohn BH, Han WK, Pae WK. Proximal gastrectomy reconstructed by jejunal pouch interposition for upper third gastric cancer: prospective randomized study. World J Surg. 2005;29:1592-1599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Kim JH, Park SS, Kim J, Boo YJ, Kim SJ, Mok YJ, Kim CS. Surgical outcomes for gastric cancer in the upper third of the stomach. World J Surg. 2006;30:1870-186; discussion 1870-186;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Nozaki I, Kurita A, Nasu J, Kubo Y, Aogi K, Tanada M, Takashima S. Higher incidence of gastric remnant cancer after proximal than distal gastrectomy. Hepatogastroenterology. 2007;54:1604-1608. [PubMed] [Cited in This Article: ] |
| 18. | An JY, Youn HG, Ha TK, Choi MG, Kim KM, Noh JH, Sohn TS, Kim S. Clinical significance of tumor location in remnant gastric cancers developed after partial gastrectomy for primary gastric cancer. J Gastrointest Surg. 2008;12:689-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | McCulloch P, Ward J, Tekkis PP. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ. 2003;327:1192-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Shibata C, Shiiba KI, Funayama Y, Ishii S, Fukushima K, Mizoi T, Koyama K, Miura K, Matsuno S, Naito H. Outcomes after pylorus-preserving gastrectomy for early gastric cancer: a prospective multicenter trial. World J Surg. 2004;28:857-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Ishikawa K, Arita T, Ninomiya S, Bandoh T, Shiraishi N, Kitano S. Outcome of segmental gastrectomy versus distal gastrectomy for early gastric cancer. World J Surg. 2007;31:2204-2207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Morita S, Katai H, Saka M, Fukagawa T, Sano T, Sasako M. Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg. 2008;95:1131-1135. [PubMed] [Cited in This Article: ] |
| 23. | Park do J, Lee HJ, Jung HC, Kim WH, Lee KU, Yang HK. Clinical outcome of pylorus-preserving gastrectomy in gastric cancer in comparison with conventional distal gastrectomy with Billroth I anastomosis. World J Surg. 2008;32:1029-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Ichikura T, Sugasawa H, Sakamoto N, Yaguchi Y, Tsujimoto H, Ono S. Limited gastrectomy with dissection of sentinel node stations for early gastric cancer with negative sentinel node biopsy. Ann Surg. 2009;249:942-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Csendes A, Burdiles P, Rojas J, Braghetto I, Diaz JC, Maluenda F. A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery. 2002;131:401-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 160] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Fatouros M, Roukos DH, Lorenz M, Arampatzis I, Hottentrott C, Encke A, Kappas AM. Impact of spleen preservation in patients with gastric cancer. Anticancer Res. 2005;25:3023-3030. [PubMed] [Cited in This Article: ] |
| 27. | Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg. 2006;93:559-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 28. | Sano T, Yamamoto S, Sasako M. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma: Japan clinical oncology group study JCOG 0110-MF. Jpn J Clin Oncol. 2002;32:363-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Martin RC, Jaques DP, Brennan MF, Karpeh M. Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Ann Surg. 2002;236:159-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Kobayashi A, Nakagohri T, Konishi M, Inoue K, Takahashi S, Itou M, Sugitou M, Ono M, Saito N, Kinoshita T. Aggressive surgical treatment for T4 gastric cancer. J Gastrointest Surg. 2004;8:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono HA, Nagahori Y, Takahashi M, Kito F, Shimada H. Surgical outcomes in patients with T4 gastric carcinoma. J Am Coll Surg. 2006;202:223-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Jeong O, Choi WY, Park YK. Appropriate selection of patients for combined organ resection in cases of gastric carcinoma invading adjacent organs. J Surg Oncol. 2009;100:115-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Colen KL, Marcus SG, Newman E, Berman RS, Yee H, Hiotis SP. Multiorgan resection for gastric cancer: intraoperative and computed tomography assessment of locally advanced disease is inaccurate. J Gastrointest Surg. 2004;8:899-902. [PubMed] [Cited in This Article: ] |
| 34. | Kim SH, Karpeh MS, Klimstra DS, Leung D, Brennan MF. Effect of microscopic resection line disease on gastric cancer survival. J Gastrointest Surg. 1999;3:24-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Cho BC, Jeung HC, Choi HJ, Rha SY, Hyung WJ, Cheong JH, Noh SH, Chung HC. Prognostic impact of resection margin involvement after extended (D2/D3) gastrectomy for advanced gastric cancer: a 15-year experience at a single institute. J Surg Oncol. 2007;95:461-468. [PubMed] [Cited in This Article: ] |
| 36. | Wang SY, Yeh CN, Lee HL, Liu YY, Chao TC, Hwang TL, Jan YY, Chen MF. Clinical impact of positive surgical margin status on gastric cancer patients undergoing gastrectomy. Ann Surg Oncol. 2009;16:2738-2743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Sun Z, Li DM, Wang ZN, Huang BJ, Xu Y, Li K, Xu HM. Prognostic significance of microscopic positive margins for gastric cancer patients with potentially curative resection. Ann Surg Oncol. 2009;16:3028-3037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069-2077. [PubMed] [Cited in This Article: ] |
| 39. | Hartgrink HH, van de Velde CJ. Status of extended lymph node dissection: locoregional control is the only way to survive gastric cancer. J Surg Oncol. 2005;90:153-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Degiuli M, Sasako M, Ponti A, Calvo F. Survival results of a multicentre phase II study to evaluate D2 gastrectomy for gastric cancer. Br J Cancer. 2004;90:1727-1732. [PubMed] [Cited in This Article: ] |
| 41. | Edwards P, Blackshaw GR, Lewis WG, Barry JD, Allison MC, Jones DR. Prospective comparison of D1 vs modified D2 gastrectomy for carcinoma. Br J Cancer. 2004;90:1888-1892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Sobin LH, Wittekind C. TNM Classification of Malignant Tumors. 5th ed. New York: John Wiley & Sons 1997; . [Cited in This Article: ] |
| 43. | Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg. 1987;11:418-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 477] [Cited by in F6Publishing: 496] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 44. | Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 461] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 45. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 698] [Cited by in F6Publishing: 727] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 46. | US National Institutes of Health. A Comparison Between D1 and D2 Lymphadenectomy in Gastric Cancer: A Prospective Randomized Controlled Trial. Available from: http: //www.clinicaltrial.gov/ct2/show/NCT00447746?term=gastric cancer surgery&rank=58. [Cited in This Article: ] |
| 47. | Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 413] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 48. | National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology, Gastric Cancer. Available from: http: //www.nccn.org/professionals/physician_gls. [Cited in This Article: ] |
| 49. | Miyata M, Yokoyama Y, Okoyama N, Joh T, Seno K, Sasaki M, Ohara H, Nomura T, Kasugai K, Itoh M. What are the appropriate indications for endoscopic mucosal resection for early gastric cancer? Analysis of 256 endoscopically resected lesions. Endoscopy. 2000;32:773-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Technology status report evaluation. Endoscopic mucosal resection. Gastrointest Endosc. 2000;52:860-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1134] [Cited by in F6Publishing: 1104] [Article Influence: 48.0] [Reference Citation Analysis (4)] |
| 52. | O'Mahony S. Endoscopic mucosal resection for early gastric cancer. Gut. 2001;48:151-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Yoshikane H, Sakakibara A, Hidano H, Niwa Y, Goto H, Yokoi T. Piecemeal endoscopic aspiration mucosectomy for large superficial intramucosal tumors of the stomach. Endoscopy. 2001;33:795-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Miyamoto S, Muto M, Hamamoto Y, Boku N, Ohtsu A, Baba S, Yoshida M, Ohkuwa M, Hosokawa K, Tajiri H. A new technique for endoscopic mucosal resection with an insulated-tip electrosurgical knife improves the completeness of resection of intramucosal gastric neoplasms. Gastrointest Endosc. 2002;55:576-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 196] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 55. | Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 296] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 56. | Tanabe S, Koizumi W, Mitomi H, Nakai H, Murakami S, Nagaba S, Kida M, Oida M, Saigenji K. Clinical outcome of endoscopic aspiration mucosectomy for early stage gastric cancer. Gastrointest Endosc. 2002;56:708-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Yamamoto H, Kawata H, Sunada K, Satoh K, Kaneko Y, Ido K, Sugano K. Success rate of curative endoscopic mucosal resection with circumferential mucosal incision assisted by submucosal injection of sodium hyaluronate. Gastrointest Endosc. 2002;56:507-512. [PubMed] [Cited in This Article: ] |
| 58. | Katsube T, Ogawa K, Hamaguchi K, Shimao K, Yamaguchi K, Konno S, Shimakawa T, Naritaka Y, Yagawa H, Aiba M. Modification of endoscopic aspiration mucosectomy (EAM) for early gastric cancer: EAM with pre-cutting. Hepatogastroenterology. 2002;49:1510-1513. [PubMed] [Cited in This Article: ] |
| 59. | Matsuzaki K, Nagao S, Kawaguchi A, Miyazaki J, Yoshida Y, Kitagawa Y, Nakajima H, Kato S, Hokari R, Tsuzuki Y. Newly designed soft prelooped cap for endoscopic mucosal resection of gastric lesions. Gastrointest Endosc. 2003;57:242-246. [PubMed] [Cited in This Article: ] |
| 60. | Eguchi T, Gotoda T, Oda I, Hamanaka MH, Hasuike N, Saito D. Is endoscopic one-piece mucosal resection essential for early gastric cancer? Digest Endosc. 2003;15:113-116. [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 61. | Oda I, Gotoda T, Hamanaka H, Eguchi T, Saito Y, Matsuda T, Bhandari P, Emura F, Saito D, Ono H. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Digest Endosc. 2005;17:54-58. [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 345] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 62. | Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 216] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 63. | Kakushima N, Fujishiro M, Kodashima S, Muraki Y, Tateishi A, Omata M. A learning curve for endoscopic submucosal dissection of gastric epithelial neoplasms. Endoscopy. 2006;38:991-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 64. | Oyama T, Tanaka M, Tomori A, Hotta K, Morita S, Furutachi S, Takahashi A, Miyata Y. Prognosis of endoscopic submucosal dissection for early gastric cancer, results of 3 years or more after treatment. Stomach Intest. 2006;41:87-90. [Cited in This Article: ] |
| 65. | Onozato Y, Ishihara H, Iizuka H, Sohara N, Kakizaki S, Okamura S, Mori M. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy. 2006;38:980-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 66. | Hirasaki S, Kanzaki H, Matsubara M, Fujita K, Ikeda F, Taniguchi H, Yumoto E, Suzuki S. Treatment of over 20 mm gastric cancer by endoscopic submucosal dissection using an insulation-tipped diathermic knife. World J Gastroenterol. 2007;13:3981-3984. [PubMed] [Cited in This Article: ] |
| 67. | Yamaguchi N, Isomoto H, Fukuda E, Ikeda K, Nishiyama H, Akiyama M, Ozawa E, Ohnita K, Hayashi T, Nakao K. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion. 2009;80:173-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 68. | Nakamoto S, Sakai Y, Kasanuki J, Kondo F, Ooka Y, Kato K, Arai M, Suzuki T, Matsumura T, Bekku D. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy. 2009;41:746-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 69. | Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, Costa N, Lopes C, Moreira-Dias L. A European case series of endoscopic submucosal dissection for gastric superficial lesions. Gastrointest Endosc. 2009;69:350-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 70. | Kakushima N, Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J Gastroenterol. 2008;14:2962-2967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 114] [Cited by in F6Publishing: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 71. | Bennett C, Wang Y, Pan T. Endoscopic mucosal resection for early gastric cancer. Cochrane Database Syst Rev. 2009;CD004276. [PubMed] [Cited in This Article: ] |
| 72. | Japanese Gastric Cancer Association. Treatment Guideline for Gastric Cancer in Japan. 2rd ed. Tokyo: Kanehara 2004; . [Cited in This Article: ] |
| 73. | Tada M, Murata M, Murakami F, Shimada M, Mizumachi S, Arima K. The development of strip-off biopsy. Gastroenterol Endosc. 1984;26:833-836. [Cited in This Article: ] |
| 74. | Tada M, Yanai H, Takemoto T. New technique of gastric biopsy. Stomach Intestine. 1984;19:1107-1116. [Cited in This Article: ] |
| 75. | Tada M, Karita M, Yanai H, Kawano H, Shigeeda . M, Fukumoto Y. Treatment of early gastric cancer using strip biopsy, a new technique for jumbo biopsy. Recent Topics of Digestive Endoscopy. Tokyo: Excerpta Medica 1987; . [Cited in This Article: ] |
| 76. | Takemoto T, Tada M, Yanai H, Karita M, Okita K. Significance of strip biopsy, with particular reference to endoscopic mucosectomy. Dig Endosc. 1989;1:4-9. [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 77. | Tada M, Murakami A, Karita M, Yanai H, Okita K. Endoscopic resection of early gastric cancer. Endoscopy. 1993;25:445-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 289] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 78. | Tada M, Tokiyama H, Nakamura H, Yanai H, Okita K. Endoscopic resection for early gastric cancer. Acta Endosc. 1998;28:87-95. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 79. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1308] [Cited by in F6Publishing: 1273] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 80. | Ishikawa S, Togashi A, Inoue M, Honda S, Nozawa F, Toyama E, Miyanari N, Tabira Y, Baba H. Indications for EMR/ESD in cases of early gastric cancer: relationship between histological type, depth of wall invasion, and lymph node metastasis. Gastric Cancer. 2007;10:35-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 81. | Ha TK, An JY, Youn HK, Noh JH, Sohn TS, Kim S. Indication for endoscopic mucosal resection in early signet ring cell gastric cancer. Ann Surg Oncol. 2008;15:508-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 82. | Park JM, Kim SW, Nam KW, Cho YK, Lee IS, Choi MG, Chung IS, Song KY, Park CH, Jung CK. Is it reasonable to treat early gastric cancer with signet ring cell histology by endoscopic resection? Analysis of factors related to lymph-node metastasis. Eur J Gastroenterol Hepatol. 2009;21:1132-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Kim JH, Lee YC, Kim H, Song KH, Lee SK, Cheon JH, Kim H, Hyung WJ, Noh SH, Kim CB. Endoscopic resection for undifferentiated early gastric cancer. Gastrointest Endosc. 2009;69:e1-e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 84. | Ida K, Nakazawa S, Yoshino J, Hiki Y, Akamatsu T, Asaki S, Kurihara M, Shimao H, Tada M, Misumi A. Multicentre collaborative prospective study of endoscopic treatment for early gastric cancer. Digest Endosc. 2004;16:295-232. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Ryu KW, Choi IJ, Doh YW, Kook MC, Kim CG, Park HJ, Lee JH, Lee JS, Lee JY, Kim YW. Surgical indication for non-curative endoscopic resection in early gastric cancer. Ann Surg Oncol. 2007;14:3428-3434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 86. | Song KY, Hyung WJ, Kim HH, Han SU, Cho GS, Ryu SW, Lee HJ, Kim MC. Is gastrectomy mandatory for all residual or recurrent gastric cancer following endoscopic resection? A large-scale Korean multi-center study. J Surg Oncol. 2008;98:6-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Kurokawa Y, Hasuike N, Ono H, Boku N, Fukuda H. A phase II trial of endoscopic submucosal dissection for mucosal gastric cancer: Japan Clinical Oncology Group Study JCOG0607. Jpn J Clin Oncol. 2009;39:464-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 88. | Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer. 1999;2:230-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 186] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 89. | Ohki J, Nagai H, Hyodo M, Nagashima T. Hand-assisted laparoscopic distal gastrectomy with abdominal wall-lift method. Surg Endosc. 1999;13:1148-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Azagra JS, Goergen M, De Simone P, Ibañez-Aguirre J. Minimally invasive surgery for gastric cancer. Surg Endosc. 1999;13:351-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 137] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 91. | Goh PM, Khan AZ, So JB, Lomanto D, Cheah WK, Muthiah R, Gandhi A. Early experience with laparoscopic radical gastrectomy for advanced gastric cancer. Surg Laparosc Endosc Percutan Tech. 2001;11:83-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Mochiki E, Nakabayashi T, Kamimura H, Haga N, Asao T, Kuwano H. Gastrointestinal recovery and outcome after laparoscopy-assisted versus conventional open distal gastrectomy for early gastric cancer. World J Surg. 2002;26:1145-1149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 93. | Tanimura S, Higashino M, Fukunaga Y, Osugi H. Laparoscopic distal gastrectomy with regional lymph node dissection for gastric cancer. Surg Endosc. 2003;17:758-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Fujiwara M, Kodera Y, Kasai Y, Kanyama Y, Hibi K, Ito K, Akiyama S, Nakao A. Laparoscopy-assisted distal gastrectomy with systemic lymph node dissection for early gastric carcinoma: a review of 43 cases. J Am Coll Surg. 2003;196:75-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 95. | Japanese Gastric Cancer Association. The guidelines for the treatment of gastric cancer. Tokyo: Kachara 2001; . [Cited in This Article: ] |
| 96. | Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131:S306-S311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 449] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 97. | Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 298] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 98. | Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172-1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 251] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 99. | Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 650] [Cited by in F6Publishing: 641] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 100. | US National Institutes of Health. A Phase III Trial of Laparoscopy-Assisted Distal Gastrectomy in Early Gastric Cancer. Available from: http: //www.clinicaltrial.gov/ct2/show/NCT00546468?term=gastric cancer surgery&rank=63. [Cited in This Article: ] |
| 101. | US National Institutes of Health. A Phase III Trial of Laparoscopy-Assisted Distal Gastrectomy in Early Gastric Cancer. Available from: http: //www.clinicaltrial.gov/ct2/show/NCT00452751?term=gastric cancer surgery&rank=27. [Cited in This Article: ] |
| 102. | Abe N, Mori T, Takeuchi H, Yoshida T, Ohki A, Ueki H, Yanagida O, Masaki T, Sugiyama M, Atomi Y. Laparoscopic lymph node dissection after endoscopic submucosal dissection: a novel and minimally invasive approach to treating early-stage gastric cancer. Am J Surg. 2005;190:496-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 103. | Huscher CG, Mingoli A, Sgarzini G, Brachini G, Binda B, Di Paola M, Ponzano C. Totally laparoscopic total and subtotal gastrectomy with extended lymph node dissection for early and advanced gastric cancer: early and long-term results of a 100-patient series. Am J Surg. 2007;194:839-44; discussion 844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 104. | Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 509] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 105. | Hur H, Jeon HM, Kim W. Laparoscopy-assisted distal gastrectomy with D2 lymphadenectomy for T2b advanced gastric cancers: three years' experience. J Surg Oncol. 2008;98:515-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 106. | Mochiki E, Toyomasu Y, Ogata K, Andoh H, Ohno T, Aihara R, Asao T, Kuwano H. Laparoscopically assisted total gastrectomy with lymph node dissection for upper and middle gastric cancer. Surg Endosc. 2008;22:1997-2002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 107. | Hwang SH, Park do J, Jee YS, Kim MC, Kim HH, Lee HJ, Yang HK, Lee KU. Actual 3-year survival after laparoscopy-assisted gastrectomy for gastric cancer. Arch Surg. 2009;144:559-64; discussion 565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 108. | Hiratsuka M, Miyashiro I, Ishikawa O, Furukawa H, Motomura K, Ohigashi H, Kameyama M, Sasaki Y, Kabuto T, Ishiguro S. Application of sentinel node biopsy to gastric cancer surgery. Surgery. 2001;129:335-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 109. | Kitagawa Y, Fujii H, Mukai M, Kubota T, Otani Y, Kitajima M. Radio-guided sentinel node detection for gastric cancer. Br J Surg. 2002;89:604-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 110. | Ajisaka H, Miwa K. Micrometastases in sentinel nodes of gastric cancer. Br J Cancer. 2003;89:676-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 111. | Ryu KW, Lee JH, Kim HS, Kim YW, Choi IJ, Bae JM. Prediction of lymph nodes metastasis by sentinel node biopsy in gastric cancer. Eur J Surg Oncol. 2003;29:895-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 112. | Kitagawa Y, Fujii H, Kumai K, Kubota T, Otani Y, Saikawa Y, Yoshida M, Kubo A, Kitajima M. Recent advances in sentinel node navigation for gastric cancer: a paradigm shift of surgical management. J Surg Oncol. 2005;90:147-51; discussion 151-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 113. | Kitagawa Y, Fujii H, Mukai M, Kubota T, Ando N, Watanabe M, Ohgami M, Otani Y, Ozawa S, Hasegawa H. The role of the sentinel lymph node in gastrointestinal cancer. Surg Clin North Am. 2000;80:1799-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 220] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 114. | Hundley JC, Shen P, Shiver SA, Geisinger KR, Levine EA. Lymphatic mapping for gastric adenocarcinoma. Am Surg. 2002;68:931-935. [PubMed] [Cited in This Article: ] |
| 115. | Orsenigo E, Tomajer V, Di Palo S, Albarello L, Doglioni C, Masci E, Viale E, Staudacher C. Sentinel node mapping during laparoscopic distal gastrectomy for gastric cancer. Surg Endosc. 2008;22:118-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2465] [Cited by in F6Publishing: 2366] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
| 117. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1771] [Cited by in F6Publishing: 1830] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 118. | House MG, Brennan MF. Neoadjuvant therapy for gastric cancer. Adv Surg. 2008;42:151-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 119. | Schuhmacher CP, Fink U, Becker K, Busch R, Dittler HJ, Mueller J, Siewert JR. Neoadjuvant therapy for patients with locally advanced gastric carcinoma with etoposide, doxorubicin, and cisplatinum. Closing results after 5 years of follow-up. Cancer. 2001;91:918-927. [PubMed] [Cited in This Article: ] |
| 120. | Persiani R, D'Ugo D, Rausei S, Sermoneta D, Barone C, Pozzo C, Ricci R, La Torre G, Picciocchi A. Prognostic indicators in locally advanced gastric cancer (LAGC) treated with preoperative chemotherapy and D2-gastrectomy. J Surg Oncol. 2005;89:227-36; discussion 237-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 121. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4120] [Cited by in F6Publishing: 4280] [Article Influence: 237.8] [Reference Citation Analysis (0)] |
| 122. | Boige V, Pignon J, Saint-Aubert B, Lasser P, Conroy T, Bouché O, Segol P, Bedenne L, Rougier P, Ychou M. Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. J Clin Oncol. 2007;25:18S (abstr 4510). [Cited in This Article: ] |
| 123. | US National Institutes of Health. A trial of neoadjuvant TS-1 and cisplatin for type 4 and large type 3 gastric cancer. Available from: http: //clinicaltrial.gov/ct2/show/NCT00252161?term=JCOG trial 0501&rank=1. [Cited in This Article: ] |
| 124. | US National Institutes of Health. Combination chemotherapy with or without bevacizumab in treating patients with previously untreated stomach cancer or gastroesophageal junction cancer that can be removed by surgery. Available from: http: //clinicaltrial.gov/ct2/show/NCT00450203? term=ST03 trial&rank=2. [Cited in This Article: ] |
| 125. | US National Institutes of Health. Multicenter Randomized Phase III Trial of Neo-Adjuvant Chemotherapy Followed by Surgery and Chemotherapy or by Surgery and Chemoradiotherapy in Resectable Gastric Cancer, CRITICS Study. Available from: http: //www.clinicaltrial.gov/ct2/show/NCT00407186?term=gastric cancer surgery&rank=16. [Cited in This Article: ] |
| 126. | Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, Greskovich JF, Anne PR, Bradley JD, Willett C. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953-3958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 127. | Wydmański J, Suwinski R, Poltorak S, Maka B, Miszczyk L, Wolny E, Bielaczyc G, Zajusz A. The tolerance and efficacy of preoperative chemoradiotherapy followed by gastrectomy in operable gastric cancer, a phase II study. Radiother Oncol. 2007;82:132-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |