Published online Apr 27, 2024. doi: 10.4240/wjgs.v16.i4.1208
Peer-review started: January 16, 2024
First decision: February 2, 2024
Revised: February 14, 2024
Accepted: March 21, 2024
Article in press: March 21, 2024
Published online: April 27, 2024
Lymphangiomas in the gastrointestinal tract are extremely rare in adults. As a benign lesion, small intestine lymphangiomas often remain asymptomatic and pose challenges for definitive diagnosis. However, lymphangiomas can give rise to complications such as abdominal pain, bleeding, volvulus, and intussusception. Here, we report a case of jejunal cavernous lymphangioma that presented with intermittent melena and refractory anemia in a male adult.
A 66-year-old man presented with intermittent melena, fatigue and refractory anemia nine months prior. Esophagogastroduodenoscopy and colonoscopy were performed many times and revealed no apparent bleeding. Conservative mana
Jejunal cavernous lymphangioma should be considered a cause of obscure gas
Core Tip: We report a patient with recurrent melena and refractory anemia that was misdiagnosed as obscure gastrointestinal bleeding or intestinal ischemia for 9 months. A definite diagnosis of jejunal cavernous lymphangioma was made through capsule endoscopy, double-balloon enteroscopy, and biopsy. After surgical resection of the lesion, both the melena and anemia resolved.
- Citation: Liu KR, Zhang S, Chen WR, Huang YX, Li XG. Intermittent melena and refractory anemia due to jejunal cavernous lymphangioma: A case report. World J Gastrointest Surg 2024; 16(4): 1208-1214
- URL: https://www.wjgnet.com/1948-9366/full/v16/i4/1208.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i4.1208
Lymphangioma is an uncommon benign neoplasm that arises from congenital dysplasia of the lymphatic system and occurs more rarely within the small intestine in adults, accounting for less than 1% of all cases[1,2]. Small bowel lymphangiomas lack characteristic manifestations and symptoms. The clinical presentations of asymptomatic chronic wasting diseases vary and include weight loss, hypoproteinemia, lymphopenia, hypogammaglobulinemia and iron deficiency anemia; acute abdominalgia, for instance, volvulus; intussusception; and acute intestinal obstruction[1,3-7]. In this report, we present a case of recurrent melena and refractory anemia caused by jejunal cavernous lymphangioma, which was ini
A 66-year-old man presented with recurrent melena and fatigue for 9 months.
The patient presented with melena approximately of unknown etiology for 3 to 4 times a day and that started nine mon
The patient had a history of hypertension, which was under control with medical treatment. He denied any history of trauma or surgery.
No hereditary diseases or history of allergies, alcoholism or epidemiologic linkage were described.
The patient presented with an anemic appearance. The abdomen was soft. No tenderness, peritonitis or palpable mass was observed. No abnormalities were detected during a digital rectal examination. Cardiopulmonary and neurological examinations revealed no abnormalities.
His hemoglobin level was 6.7 g/dL, and his fecal occult blood test was strongly positive at admission. The C-reactive protein level was 11.7 mg/L. His leukocyte count and procalcitonin level were normal. The lymphocyte count and lymphocyte percentage were 0.47 × 109/L and 14.6%, respectively. The thrombocyte count and coagulation function were also within the normal range. No positive laboratory results were detected for carcinoembryonic antigen, cancer antigen 19-9, tuberculosis antibody, autoimmune disease-related antibodies, immunoglobulin A (IgA), IgG, or IgM. There were no abnormalities in liver or renal function tests.
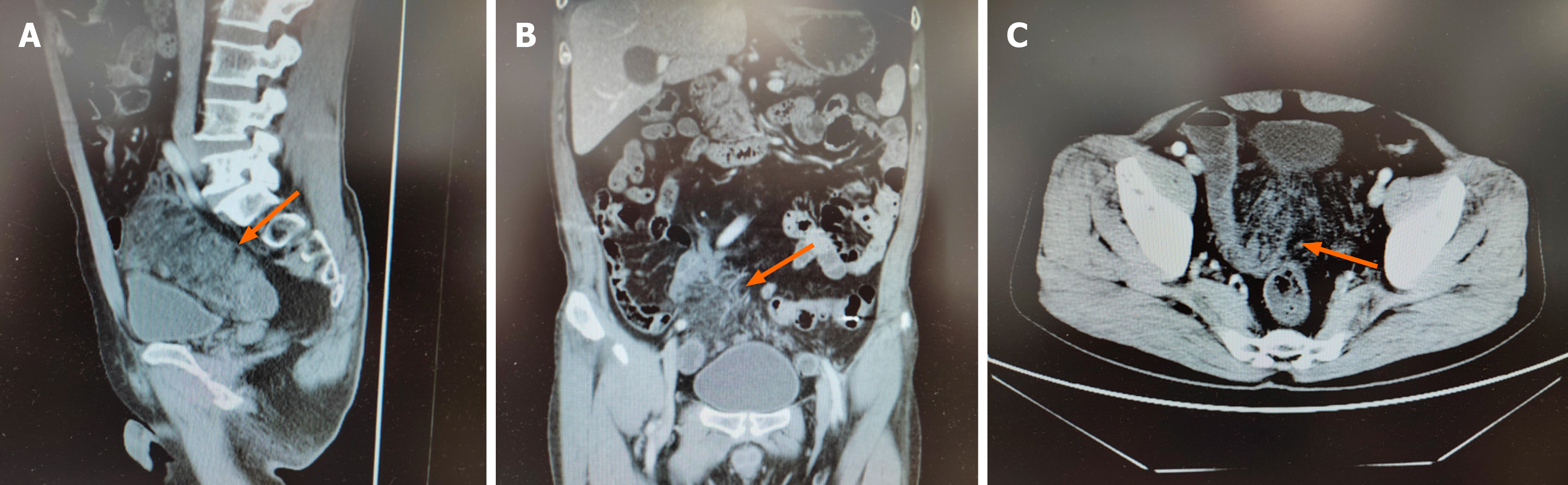
No hemorrhagic foci were detected during esophagogastroduodenoscopy or colonoscopy. Contrast-enhanced computed tomography (CT) of the entire abdomen revealed ischemic changes on the mesenteric side of the distal jejunal and ad
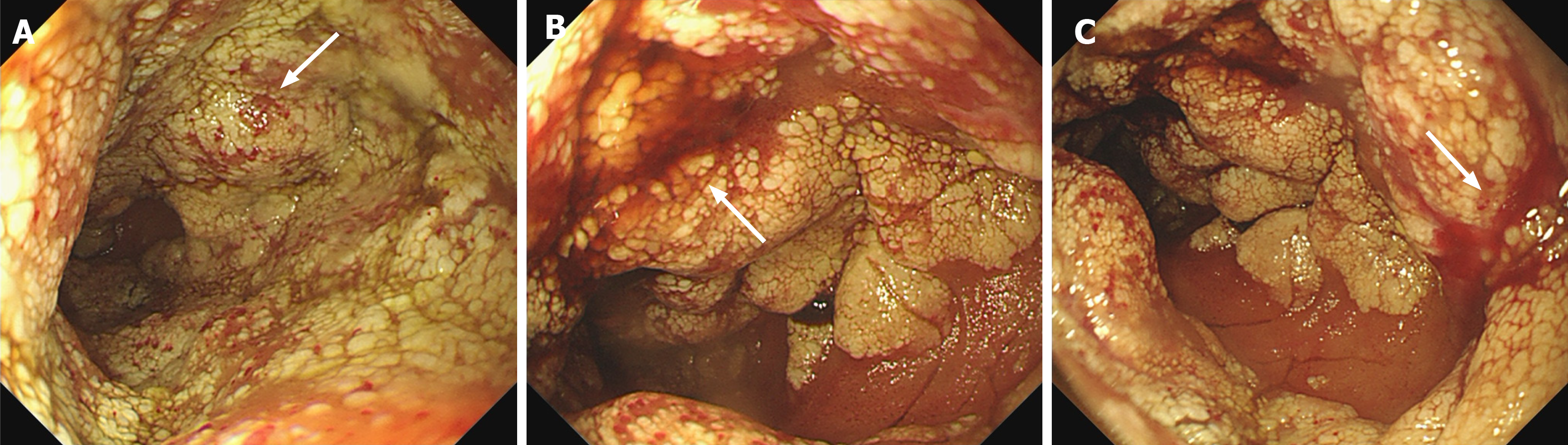
According to the enteroscopy findings and pathological results, a definitive diagnosis of jejunal cavernous lymphangioma was established.
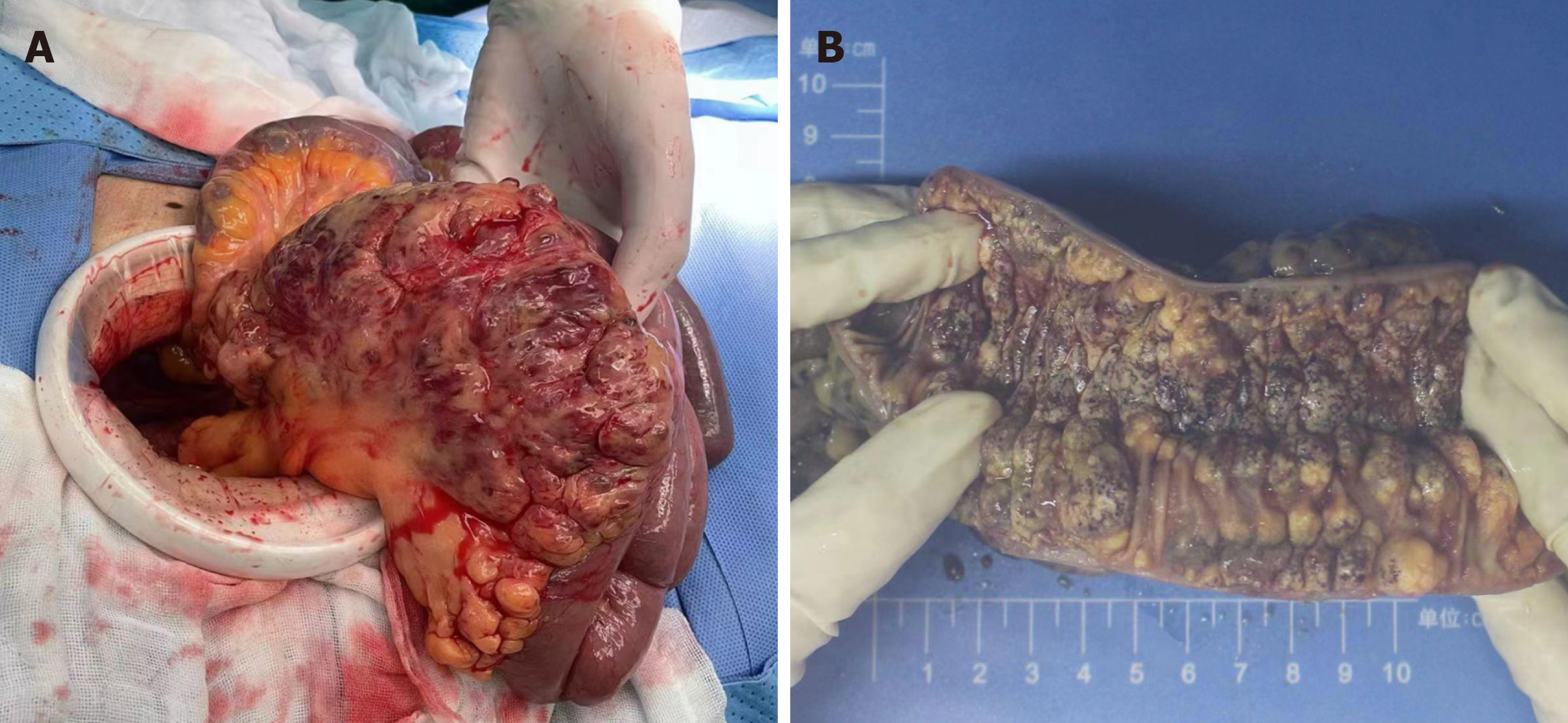
After positive preoperative preparation, exploratory laparotomy was performed. During intraoperative exploration, edema and thickening of the intestinal wall were observed approximately 2 meters away from the Treitz ligament. The length of the diseased jejunum exceeded 10 cm. Numerous cystic and hemorrhagic masses of varying sizes were observed on the mesentery, and these lesions were infiltrating into the intestinal lumen (Figure 4). The patient underwent resection of the diseased jejunum and mesentery, followed by side-to-side jejunal anastomosis. Following a three-day fasting pe
The anemia resolved, and 4 months after the operation, his hemoglobin level increased to 12.9 g/dL without transfusion. No complications or recurrences were observed during the 6-month follow-up period.
Lymphangioma can arise from malformations of the lymphatic system during embryonic development or from acquired factors such as trauma, surgery, inflammation, and parasitic infections[8]. Lymphangioma can be histopathologically classified into three subtypes: Cavernous, capillary, and cystic lymphangioma, which may manifest in any part of the body[9,10]. The preferential localization of the fungus is predominantly in the head, neck, and axilla, encompassing ap
There is no characteristic clinical presentation of lymphangioma. The manifestation may be asymptomatic at times and can occasionally encompass a range of symptoms, including abdominal pain, melena, and acute peritonitis. Hemorrhage, perforation, torsion, intussusception, and rupture are frequent causes of hospital admission[16]. Occult gastrointestinal bleeding or obscure intermittent melena may be the predominant clinical manifestations, accounting for more than half of the cases (28 out of 52, 53.8%) reported in the English literature. The mechanism of bleeding may be attributed to the obstruction of lymphatic flow, resulting in increased pressure on lymphatic–venous connections and subsequent retro
The laboratory tests conducted for lymphangiomas lack specificity. In this case, we observed a mild decrease in the lymphocyte count, which has been previously reported in two similar cases[3,4]. It is speculated that lymphopenia may serve as one of the clinical features associated with lymphangioma. However, the underlying mechanism is unclear. One postulation is that this condition could be due to the excessive loss of lymphatic fluid containing lymphocytes into the intestinal lumen[24].
The use of CT, magnetic resonance imaging (MRI), and ultrasound, although lacking specificity and having limited accuracy, is advantageous for facilitating the detection of lesions and differential diagnosis. On CT, intestinal lymphangiomas are nonenhanced, well-demarcated and low-density oval masses beneath the submucous membrane[8]. CT scans may yield inconclusive results, with a positive rate of 39.1%[3]. However, it plays a crucial role in the detection of masses that manifest as asymptomatic large abdominal masses or those that give rise to complications, such as perforation, volvulus, and intussusception. MRI scans often reveal a prolonged T1 and T2 signal, a high compression lipid signal and homogeneous/hypodense lesions[22]. Ultrasound can detect multiple cystic anechoic-hypoechoic masses that exhibit thickening of the mucosal and submucosal layers and that display a hypoechoic heterogeneous component[15]. Positron emission tomography/CT is helpful for identifying malignant tumors.
The utilization of capsule endoscopy may represent one of the most efficacious approaches for the diagnosis of lym
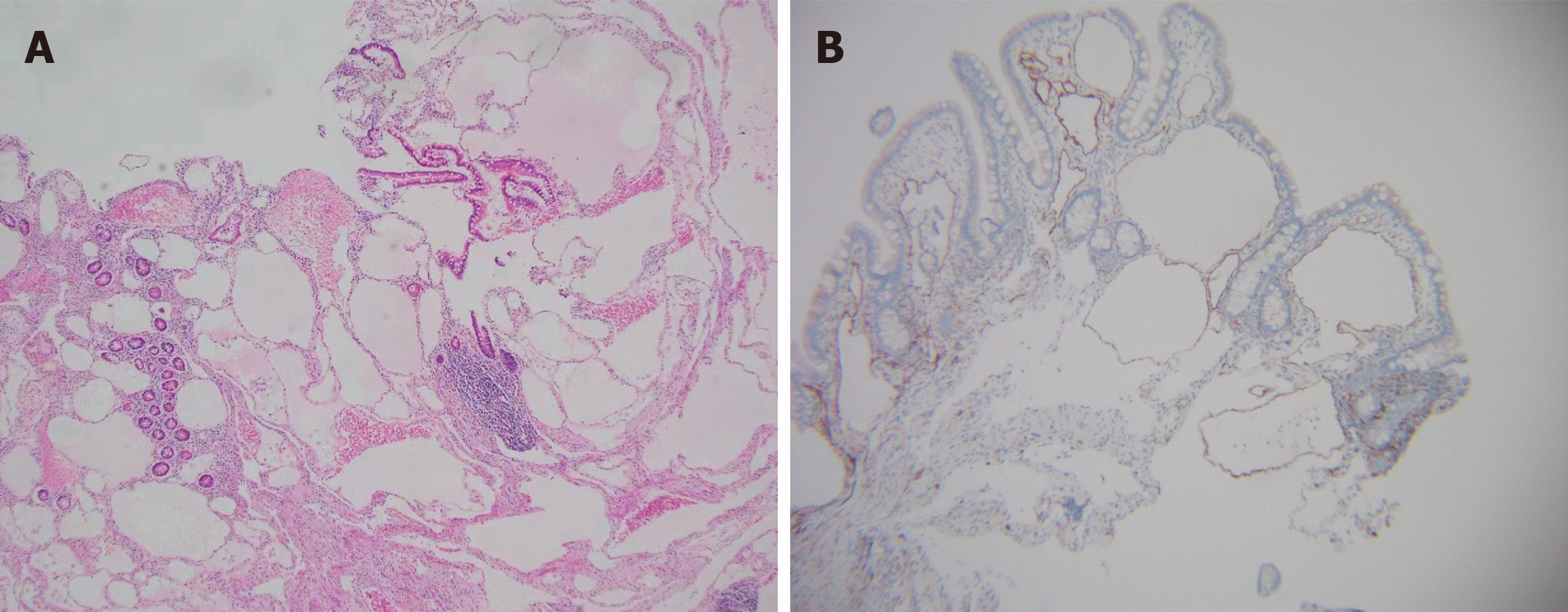
Histopathologic presentation of cavernous lymphangioma revealed large dilated and congested lymphatic lumens expanding to the mucosa of the intestine[6,23]. D2-40, CD31, VEGFR3 and ERG expression is valuable for demonstrating lymphangioma endothelial cells[4,25,26]. The expression of CD34 can serve as a reliable marker for distinguishing lym
Specific interventions are rarely necessary for asymptomatic lymphangiomas, whereas surgical resection remains the established approach for individuals experiencing symptoms.
Recently, compelling evidence has demonstrated the feasibility, efficacy, and safety of endoscopic resection for patients with lymphangiomas smaller than 2 cm[16,18,19,21,28]. Conservative therapies, such as cryotherapy, laser therapy, and local administration of sclerosing agents, are disfavored[29]. The implementation of meticulous follow-up is crucial, as recurrence rates range from approximately 10% to 27% for completely excised lesions and from 50% to 100% for partially resected lesions[13,29].
Jejunal cavernous lymphangioma, albeit rare, should be considered one of the etiologies of obscure gastrointestinal bleeding. Capsule endoscopy and single-balloon enteroscopy play pivotal roles in facilitating diagnosis. Surgical re
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sanayeh EB, United States S-Editor: Fan JR L-Editor: A P-Editor: Xu ZH
| 1. | Kohga A, Kawabe A, Hasegawa Y, Yajima K, Okumura T, Yamashita K, Isogaki J, Suzuki K, Komiyama A. Ileo-ileal intussusception caused by lymphangioma of the small bowel treated by single-incision laparoscopic-assisted ileal resection. World J Gastroenterol. 2017;23:167-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Akashige T, Sato K, Odajima H, Yamazaki S. A case report of recurrent intussusception caused by small bowel lymphangioma in an adult. Int J Surg Case Rep. 2020;75:126-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Tan B, Zhang SY, Wang YN, Li Y, Shi XH, Qian JM. Jejunal cavernous lymphangioma manifested as gastrointestinal bleeding with hypogammaglobulinemia in adult: A case report and literature review. World J Clin Cases. 2020;8:140-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Ilhan M, Oner G, Alibeyoglu A, Yeğen G, Gök AF, Akyüz F, Bicen F. Primary intestinal lymphangiomatosis of the ileum in an adult-the role of surgical approach. J Surg Case Rep. 2016;2016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Alfadhel SF, Alghamdi AA, Alzahrani SA. Ileal volvulus secondary to cystic lymphangioma: A rare case report with a literature review. Avicenna J Med. 2019;9:82-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Giuliani A, Romano L, Coletti G, Walid A Fatayer M, Calvisi G, Maffione F, Muolo C, Vicentini V, Schietroma M, Carlei F. Lymphangiomatosis of the ileum with perforation: A case report and review of the literature. Ann Med Surg (Lond). 2019;41:6-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Samuelson H, Giannotti G, Guralnick A. Jejunal lymphangioma causing intussusception in an adult: An unusual case with review of the literature. Ann Med Surg (Lond). 2018;34:39-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Wang ZZ, Shen LY, Zhou JJ, Tang JL, Ye LP, Shen CB, Li SW, Zhou XB. Clinical manifestation and treatment of small intestinal lymphangioma: A single center analysis of 15 cases. Front Med (Lausanne). 2022;9:975698. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 9. | Campbell WJ, Irwin ST, Biggart JD. Benign lymphangioma of the jejunal mesentery: an unusual cause of small bowel obstruction. Gut. 1991;32:1568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Chen CW, Hsu SD, Lin CH, Cheng MF, Yu JC. Cystic lymphangioma of the jejunal mesentery in an adult: a case report. World J Gastroenterol. 2005;11:5084-5086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in F6Publishing: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Fluke LM, Bamberger PK. Cystic Lymphangiomas: Unusual Findings Resulting in Common General Surgery Presentations. Am Surg. 2018;84:e262-e264. [PubMed] [Cited in This Article: ] |
| 12. | Wei MY, Chua J, Cheng Y, Grossberg P. Small bowel volvulus in an adult with mesenteric lymphangioma and ascariasis. ANZ J Surg. 2018;88:E859-E860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Teng Y, Wang J, Xi Q. Jejunal hemolymphangioma: A case report. Medicine (Baltimore). 2020;99:e18863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Costa JM, Carvalho SD, Gonçalves R, Mascarenhas-Saraiva M, Soares JB. Small bowel lymphangioma. Clin Res Hepatol Gastroenterol. 2019;43:120-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Tanaka Y, Fujii S, Kusaka T, Kokuryu H. Effective use of EUS for diagnosing a jejunal lymphangioma accompanied with hemorrhage. Gastrointest Endosc. 2020;91:199-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Wu TL, Hsu HT, Yen HH. Jejunum lymphangioma: a rare case of obscure gastrointestinal bleeding with successful endoscopic therapy. Endoscopy. 2021;53:E307-E308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Yang J, Zhang Y, Kou G, Li Y. Jejunum Hemolymphangioma Causing Refractory Anemia in a Young Woman. Am J Gastroenterol. 2020;115:810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Ito Y, Osuga T, Kawasaki K, Ikura Y. Jejunal hemorrhage due to hemolymphangioma successfully detected and controlled by double-balloon enteroscopy. Clin Case Rep. 2021;9:e05153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | João M, Gravito-Soares E, Lopes S, Amaro P. Jejunal cavernous lymphangioma: successful endoscopic treatment of a rare cause of small bowel bleeding. Ann Gastroenterol. 2021;34:891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Hokama A, Iraha A. Jejunal lymphangioma. Rev Esp Enferm Dig. 2023;115:103-104. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 21. | Trieu J, Dua A, Gupta N, Venu RP, Venu M. Single-Balloon-Assisted Enteroscopy With Endoscopic Mucosal Resection of a Bleeding Jejunal Lymphangioma. ACG Case Rep J. 2022;9:e00860. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 22. | Zhao N, Fu Y, Wang Z, An Q, Jia W. Case report: Submucosal cavernous lymphangioma causing jejuno-jejunal intussusception in an adult. Front Surg. 2022;9:953840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Xu J, Zhang C, Tang C. A case of jejunal hemolymphangioma. Dig Liver Dis. 2023;55:291-293. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 24. | Amadori G, Micciolo R, Poletti A. A case of intra-abdominal multiple lymphangiomas in an adult in whom the immunological evaluation supported the diagnosis. Eur J Gastroenterol Hepatol. 1999;11:347-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Fukunaga M. Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology. 2005;46:396-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 26. | Galambos C, Nodit L. Identification of lymphatic endothelium in pediatric vascular tumors and malformations. Pediatr Dev Pathol. 2005;8:181-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Lee YS, Kim GW, Cho HJ, Shim CS. Colonic lymphangiomatosis resolved after excisional biopsy. Clin Endosc. 2015;48:81-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Hizawa K, Matsumoto T, Kouzuki T, Suekane H, Esaki M, Fujishima M. Cystic submucosal tumors in the gastrointestinal tract: endosonographic findings and endoscopic removal. Endoscopy. 2000;32:712-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Kosmidis I, Vlachou M, Koutroufinis A, Filiopoulos K. Hemolymphangioma of the lower extremities in children: two case reports. J Orthop Surg Res. 2010;5:56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |