Published online Apr 27, 2024. doi: 10.4240/wjgs.v16.i4.1155
Peer-review started: December 28, 2023
First decision: January 16, 2024
Revised: January 26, 2024
Accepted: February 25, 2024
Article in press: February 25, 2024
Published online: April 27, 2024
The quality-adjusted life year (QALY) is a metric that is increasingly used today in the field of health economics to evaluate the value of different medical treatments and procedures. Surgical waiting lists (SWLs) represent a pressing problem in public healthcare. The QALY measure has rarely been used in the context of surgery. It would be interesting to know how many QALYs are lost by patients on SWLs.
To investigate the relationship between QALYs and SWLs in a systematic review of the scientific literature.
The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement. An unlimited search was carried out in PubMed, updated on January 19, 2024. Data on the following variables were investigated and analyzed: Specialty, country of study, procedure under study, scale used to measure QALYs, the use of a theoretical or real-life model, objectives of the study and items measured, the economic value assigned to the QALY in the country in question, and the results and conclusions publi
Forty-eight articles were selected for the study. No data were found regarding QALYs lost on SWLs. The specialties in which QALYs were studied the most in relation to the waiting list were urology and general surgery, with 15 articles each. The country in which the most studies of QALYs were carried out was the United States (n = 21), followed by the United Kingdom (n = 9) and Canada (n = 7). The most studied procedure was organ trans
The relationship between QALYs and SWLs has only rarely been studied in the literature. The rate of QALYs lost on SWLs has not been determined. Future research is warranted to address this issue.
Core Tip: This review determined that the quality-adjusted life years (QALYs) lost on surgical waiting lists (SWLs) have not been evaluated in the literature. The relationship between QALYs and SWLs has been described mainly in organ transplantation and in experimental models. The willingness-to-pay per QALY gained ranged from $100000 in the United States to €20000 in Spain. Future research should address this question, as the information recorded is likely to be of value to health systems that are planning investments aimed at reducing SWLs and cutting costs.
- Citation: de la Plaza Llamas R, Ortega Azor L, Hernández Yuste M, Gorini L, Latorre-Fragua RA, Díaz Candelas DA, Al Shwely Abduljabar F, Gemio del Rey IA. Quality-adjusted life years and surgical waiting list: Systematic review of the literature. World J Gastrointest Surg 2024; 16(4): 1155-1164
- URL: https://www.wjgnet.com/1948-9366/full/v16/i4/1155.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i4.1155
The quality-adjusted life year (QALY) is a tool that is increasingly being used in the field of health economics to assess the value of different medical treatments and procedures[1]. The determination of QALYs involves the evaluation of a patient’s quality of life based on their health status. The most common way to calculate QALYs is through the use of quality-of-life assessment questionnaires, such as the European quality of life-5 dimensions (EuroQol-5D)[2] or the short-form six-dimension survey[3]. These questionnaires assess various aspects of the patient’s quality of life and assign a score to each one. The total score obtained is converted into a QALY score using a formula that multiplies the patient’s length of life by the quality-of-life score.
For example, if a patient lives 10 years with a particular medical condition and their total quality of life score is 0.5 on a scale from 0 (death) to 1 (perfect health), and they undergo a treatment that achieves a quality of life of 1 for a period of 2 years, then they will have gained one QALY (2 years × 0.5 quality of life score) and would have lost five QALYs during the 10 years without treatment (10 years × 0.5)[4]. However, the health system does not pay for the gain in QALYs based on a previously stipulated price.
Although QALYs are widely used in decision-making regarding the allocation of resources in the health system, they have received criticism on methodological grounds and with regard to their application in decision-making concerning access to treatment. QALYs have mostly been used to evaluate the effectiveness of pharmacological treatments, and their application in the field of surgery is limited. Given that surgical waiting lists (SWLs) constitute a major problem in many public healthcare systems around the world, the time that patients remain on SWLs represents an enormous loss of QALYs, which may have profound ethical, social, and economic consequences in addition to the considerable suffering that it caused to individual patients.
Therefore, the need arises to investigate the relationship between QALYs and SWLs. Information on the QALYs lost on SWLs would encourage investment in order to shorten SWLs and thus improve patients’ quality of life and reduce costs. By examining studies that focus on this relationship, we aimed to determine the following: The QALYs lost on SWLs; the specialties and surgical procedures in which the QALY metric has been used; the models applied; the quality surveys used to specify QALYs; the countries in which they have been studied; and the willingness-to-pay (WTP) per QALY in the various studies.
Systematic review of the literature published on QALYs and SWLs. The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement[5]. The clarifications published with regard to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement were consulted[6]. There is no review protocol that can be accessed.
An unlimited search was carried out in PubMed, updated on January 19, 2024, with the following search terms: [(Quality-Adjusted Life Year) OR (QALY)] AND (Surgery) AND [(Waiting list) OR (Waitlist)].
Two independent authors screened the titles and abstracts to select the articles for inclusion. After this first selection, the entire article was read to establish whether the inclusion criteria were met.
Articles of interest had to include the following features: A theoretical or empirical approach; surgical treatment; patients on SWLs; and measurement of QALYs.
The following types of articles were excluded: Reviews of any type; meta-analyses; editorials/letters; case reports; posters; conferences; and studies involving animal models.
Data were extracted from the articles included in the review. No process automation tool was used; data from the full texts were recorded on a structured extraction Excel sheet. The main summary measure was the numerical frequency.
Data were investigated and analyzed for the following variables: Specialty; country; name of journal; study procedure; scale used to measure QALYs; nature of model (theoretical or real); the objectives of the study and the items measured; the economic value given to QALYs in that country; and the results and conclusions of the articles. The data obtained were evaluated and summarized in tables to allow comparison and interpretation.
Due to the substantial heterogeneity between the studies a meta-analysis was ruled out, but a descriptive analysis of all studies was performed. In some cases, studies were grouped together under common characteristics to provide an overview of the body of evidence and to allow a descriptive synthesis of the results. An assessment of the risk of bias was considered unnecessary nor was it appropriate to perform sensitivity or subgroup analyses.
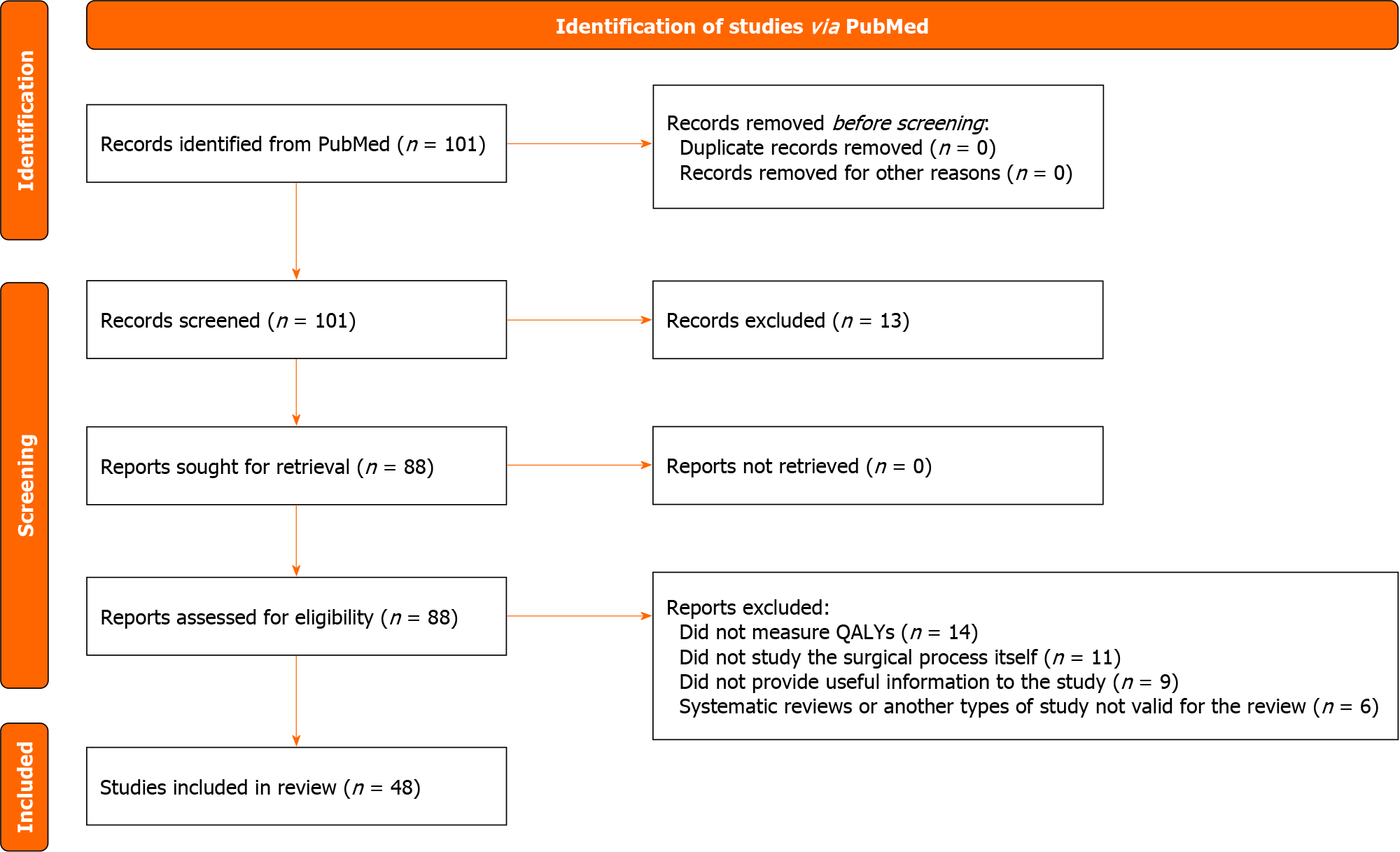
Initially 101 articles were obtained, of which 48 met the inclusion criteria and were selected for the systematic review (Figure 1). No data were found to determine the QALYs lost on different SWLs.
The specialties in which QALYs were assessed most frequently in relation to the waiting lists were urology and general surgery, both with 15 articles. In urology, kidney transplantation was the only procedure assessed; in general surgery, 14 examined liver transplantation and the other bariatric surgery. In all, 39 articles focused on organ transplantation (Table 1).
| Surgical specialties | Articles, n | Procedure evaluated and references |
| General surgery | 15 | Bariatric surgery (n = 1)[25] |
| Liver transplant (n = 14)[10,13,14,16,17,31-39] | ||
| Urology | 15 | Kidney transplant (n = 15)[11,18,40-52] |
| Heart surgery | 5 | Heart transplant (n = 4)[7-9,53] |
| Transcatheter aortic valve implantation (n = 1)[28] | ||
| Thoracic surgery | 4 | Lung transplant (n = 4)[15,54-56] |
| Traumatology | 4 | Total knee arthroplasty (n = 3)[20-22] |
| Mosaicplasty (n = 1)[27] | ||
| Pediatric surgery | 2 | Heart transplant (n = 1)[57] |
| Intestinal transplant (n = 1)[58] | ||
| Ophthalmology | 2 | Cataract surgery (n = 2)[23,24] |
| Otorhinolaryngology | 1 | Septoplasty (n = 1)[26] |
The studies were divided into two groups, depending on the type of model used to analyze the surgery: (1) Models that examined real-life procedures (n = 9); and (2) Theoretical models (n = 39) (Table 2). In the remaining nine publications the model was not specified. The type of probabilistic model used in almost all cases was the Markov model (n = 34).
| Models used | Articles, n | Type of study[references] | Articles, n |
| Real-life | 9 | ||
| Retrospective cohort[27] | 1 | ||
| Randomized controlled trial[24] | 1 | ||
| Historical and concurrent cohort[54] | 1 | ||
| Prospective randomized study[21] | 1 | ||
| Not specified[24,31,33,55,56] | 5 | ||
| Theoretical | 39 | ||
| Markov model[9-11,13-18,20,22,23,25,28,32,34-40,42-45,47-52,57,58] | 34 | ||
| Double Queueing Model[41] | 1 | ||
| Not specified[7,8,46,53] | 4 |
Surveys were used in order to assess health-related quality of life in the real and theoretical models to determine the QALYs. The most frequently used survey in both types of models was the EuroQol-5D (n = 11), followed by the 15 instrument (n = 2). However, 32 of the articles did not specify the survey used in the calculation (Table 3).
The United States was the country with the highest number of studies of QALYs carried out (n = 21), followed by the United Kingdom (n = 9) and Canada (n = 7). None of the other countries presented more than two studies (Table 4).
| Country | Articles, n[references] | Economic value of the QALY (n = 24) |
| United States | 21[7-10,13,16,22,34,37,38,40-44,47,49-51,53,57] | WTP $50000 to $100000/QALY[7-10,13,22,53,57] |
| United Kingdom | 9[14,15,24,26,27,33,39,46,56] | WTP £20000 to £30000/QALY[14,15,24,26,27,33] |
| Finland | 2[21,31] | Not specified |
| Spain | 1[28] | WTP 20000 to 25000€/QALY[28] |
| Italy and United States | 1[35] | WTP $50000/QALY[35] |
| Norway | 1[32] | WTP 70500€/QALY[32] |
| Portugal | 1[55] | WTP 50000€/QALY[55] |
| Sweden | 1[25] | WTP < €35500/QALY[25] |
| Switzerland | 1[17] | WTP €35000/QALY[17] |
To determine whether a procedure was cost-effective, the WTP threshold per QALY gained was measured in 24 studies (Table 4) and established for each country. The analysis showed that the United States was the country with the highest WTP threshold per QALY gained, at $100000/QALY. European countries in general had much lower thresholds, between €20000-€35500/QALY, with the exception of Norway, which recorded a WTP threshold of €70500/QALY gained (Table 4).
The objectives of the studies were grouped into three categories (Table 5). As the most frequently assessed procedure was organ transplantation (liver, kidney, heart, and lung), the majority of the studies (n = 26) aimed to determine whether increasing the pool of organs through various strategies was cost-effective, always taking into account the impact on QALYs and SWLs. Other studies aimed simply to see whether a specific surgical procedure was cost-effective in a certain disease or dependent on certain characteristics of the cohort in which it was performed (n = 14). Finally, there were studies that compared either two surgical procedures, a surgical procedure vs standard treatment, a bridging treatment while on the waitlist, or an alternative treatment in order to determine which had better outcomes in terms of cost-effectiveness (n = 8).
| Aim | Articles, n[references] |
| To determine whether a specific surgical procedure was cost-effective in a certain disease or dependent on certain characteristics of the cohort in which it was performed | 14[21-26,28,31-33,41,54,55,58] |
| To determine whether increasing the pool of organs through various strategies was cost-effective | 26[8-11,13-18,20,34,36-41,46-52,57] |
| To compare either two surgical procedures, a surgical procedure vs standard treatment, bridging treatment while on the waitlist, or an alternative treatment in order to determine which had better outcomes in terms of cost-effectiveness | 8[27,35,42-45,53,56] |
Given the very heterogeneous results it was decided to evaluate each study individually within the three categories in order to answer the questions addressed in this systematic review. For the most part, the surgical processes studied were considered effective since they increased QALYs and, in many cases, shortened waiting lists. For example, six of the studies aimed to measure the cost-effectiveness of increasing the organ pool by accepting the transplant of a hepatitis C virus-positive organ in hepatitis C virus-negative recipients, with appropriate antiviral treatment. All the studies reported increases in QALYs and reductions of the waiting lists with this strategy and considered it to be cost-effective despite the added cost of the antivirals[7-12]. Another strategy for expanding the organ pool involved the introduction of organs from cardiac death donors as well as from brain dead donors. This practice reduced waiting lists in all cases[13].
Two studies assessed the value of preserving and transporting organs using modern devices and techniques in preference to cold storage, such as OrganOx in liver transplantation and the ExVivo lung perfusion technique for lung transplantation. In both cases, QALYs increased and waiting times were reduced[14,15].
Four other studies evaluated the possibility of increasing the organ pool by paying live donors. Although the impact was uncertain in all four articles, the authors concluded that it might be cost-effective[16-19].
The rest of the studies presented very diverse results due to the differences in approaches and objectives.
The objectives of the present systematic review were only partially met. This was mainly due to the heterogeneity of the results obtained in the different studies, which made it difficult to draw direct comparisons or obtain firm conclusions. Although most studies recorded gains in QALYs with the measures used, none of them quantified the QALYs lost in the SWLs.
The relationship between QALYs and SWLs was assessed in eight surgical specialties. The largest number of studies performed were in the fields of digestive surgery and urology. Thirty-nine articles focused on organ transplantation; in the remaining nine, the procedures described were total knee arthroplasty (n = 3)[20-22], cataract surgery (n = 2)[23,24], bariatric surgery (n = 1)[25], septoplasty (n = 1)[26], mosaicplasty (n = 1)[27], and transcatheter aortic valve implantation (n = 1)[28]. Obviously, the focuses of these studies reflect a serious deficit in the assessment of QALYs in relation to SWLs since hardly any of the most frequent surgeries in the various specialties were represented. Despite the heterogeneity of the results, the majority of the surgeries evaluated were found to be cost-effective given that increases in QALYs and reductions in waiting lists were achieved.
Another aim of the study was to identify the models used in the studies. Theoretical models were applied in 39 of the 48 studies. Theoretical models use data already available in the literature or in the country’s national health system database to create probabilistic models able to explain the evolution of QALYs and SWLs. Theoretical models require less financial investment, are adaptable to a wide variety of contexts, and are more controlled, allowing researchers to manipulate variables in a safe environment. However, they present certain disadvantages as well. For instance, their ability to generalize results to real-world situations may be limited. They may tend toward simplification, leading to an incomplete or inaccurate understanding of the real world. They also may lack ecological validity and may be unable to provide an accurate reflection of the situation.
The theoretical model used in all studies except one was the Markov model. Markov models are widely used in studies of health economics to estimate the cost-effectiveness of specific interventions, insofar as they allow researchers to represent the evolution of diseases over time and to evaluate the effects of different treatments on this evolution in terms of cost-effectiveness (the aim of the studies analyzed)[29]. In one case, the double queuing model was used[30]. Researchers use this latter model to evaluate the cost-effectiveness of different treatment options and to determine how long patients must wait before receiving treatment. It is also used to calculate how much money should be spent on treatments compared to other health care options.
A further aim of the study was to identify the quality surveys used to calculate QALYs. Little information on this issue was forthcoming since the survey was not specified in 31 of 39 studies with a theoretical model and was not specified in 1 of the 9 real-world models. The most frequently used survey was EuroQol-5D, applied in 11 studies, which consists of five dimensions: Mobility; self-care; daily activities; physical pain/discomfort; and anxiety/depression. These five health states are combined to generate a health-related quality of life index, which ranges between -0.59 (worst possible health status) and 1 (best health status)[2].
Finally, the study also identified the countries where the studies had been carried out and if specified the WTP threshold for each QALY gained. These results regarding WTP are interesting because they highlight differences in values and priorities. They also indirectly reflect the differences in health systems and the availability of resources in each country. The United States was the country that led both in terms of number of studies (21) and in terms of WTP threshold ($50000 to $100000/QALY). The United Kingdom had nine studies and a WTP of £20000 to £30000/QALY. In the United Kingdom, extensive use is made of QALYs in the resource allocation decision-making process. Naturally, all the studies are influenced by the health data and the economy of the country in which they were conducted, and these disparities may be considered a limitation of the present study. Another limitation is the lack of information on the quality of some of the studies evaluated and on the methodology used.
It should be noted that the initial purpose of this study was to evaluate the QALYs lost by patients on SWLs. Surprisingly, however, the results in this regard provided little information. It is important to continue researching this topic to obtain more precise data regarding the impact of SWLs on the health of the population and the economy. This is why research on QALYs lost on SWLs could offer valuable opportunities for health systems to identify areas in need of improvement and thus enhance the efficiency of medical care and the management of SWLs. Future research addressing this topic is clearly warranted.
It is important to keep in mind that the measurement of QALYs has limitations and has attracted criticism since quality of life is a subjective measure that varies from person to person and may be influenced by the scale used. Likewise, the use of QALYs as a measure of effectiveness may neglect certain groups of patients whose quality of life or life expectancy are not those of the general population. With these caveats in mind, it is nonetheless clear that research into QALYs and their effect on waiting lists can help to improve medical care and ensure a more equitable distribution of limited resources.
The relationship between QALYs and SWLs has only rarely been studied in the literature. Organ transplant is the most frequently evaluated procedure, and the models used have tended to be theoretical. QALYs lost on SWLs have not been accurately determined. Further research into this question is now required because the results it produces are likely to provide valuable information for health systems seeking to reduce SWLs and save costs.
The quality-adjusted life year (QALY) is a measure that is being increasingly used in the field of health economics to assess the value of different medical treatments and procedures. Surgical waiting lists (SWL) represent a significant health problem and cause a negative effect on patients’ quality of life and an incalculable social and economic cost.
It would be useful to quantify the QALYs lost in SWLs. This information would help guide healthcare managers’ decisions regarding the allocation of economic resources in the attempt to reduce surgical waiting time in the most cost-effective manner.
The primary aim was to quantify the QALYs lost on SWLs. The secondary aims were to identify: (1) The specialties and surgical procedures in which the QALY metric has been used; (2) The models applied; (3) The quality surveys used to specify QALYs; (4) The countries in which they have been studied; and (5) The willingness-to-pay per QALY.
Systematic review of the literature published on QALYs and SWLs. The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement. An unlimited search was carried out in PubMed, updated on January 19, 2024, with the following search terms: [(Quality-Adjusted Life Year) OR (QALY)] AND (Surgery) AND [(Waiting list) OR (Waitlist)]. Two independent authors screened the titles and abstracts to select the articles for inclusion. After this first selection, the entire article was read to establish whether it met the inclusion criteria.
Forty-eight articles were selected for the study. No data were found regarding QALYs lost on SWLs. The specialties in which QALYs were studied the most in relation to the waiting lists were urology and general surgery, with 15 articles each. The country in which the most studies of QALYs were carried out was the United States (n = 21). The most studied procedure was organ transplantation (n = 39). Thirty-nine of the models used were theoretical, and nine were real-life. The survey used to measure quality of life in 11 articles was the European quality of life-5 dimensions, but in 32 articles the survey was not specified. The willingness-to-pay per QALY gained ranged from $100000 in the United States to €20000 in Spain.
QALYs lost on SWLs have not been accurately determined. The relationship between QALYs and SWLs has only rarely been studied in the literature. Organ transplantation is the most frequently evaluated procedure, and the models used have tended to be theoretical.
Studies investigating the QALYs that are lost in SWLs are now a priority. The data they provide can improve the distribution of public resources in the attempts to shorten SWLs, reduce costs, and guarantee the provision of quality healthcare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Machado NC, Brazil S-Editor: Liu H L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296:716-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1373] [Cited by in F6Publishing: 1133] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 2. | EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10083] [Cited by in F6Publishing: 10844] [Article Influence: 318.9] [Reference Citation Analysis (0)] |
| 3. | Kharroubi SA, Brazier JE, Roberts J, O'Hagan A. Modelling SF-6D health state preference data using a nonparametric Bayesian method. J Health Econ. 2007;26:597-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Gold MR, Stevenson D, Fryback DG. HALYS and QALYS and DALYS, Oh My: similarities and differences in summary measures of population Health. Annu Rev Public Health. 2002;23:115-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 399] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 5. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 728] [Cited by in F6Publishing: 2730] [Article Influence: 910.0] [Reference Citation Analysis (0)] |
| 6. | Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2580] [Cited by in F6Publishing: 2938] [Article Influence: 979.3] [Reference Citation Analysis (0)] |
| 7. | Woolley AE, Gandhi AR, Jones ML, Kim JJ, Mallidi HR, Givertz MM, Baden LR, Mehra MR, Neilan AAM. The Cost-effectiveness of Transplanting Hearts From Hepatitis C-infected Donors Into Uninfected Recipients. Transplantation. 2023;107:961-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 8. | Wayda B, Sandhu AT, Parizo J, Teuteberg JJ, Khush KK. Cost-effectiveness and system-wide impact of using Hepatitis C-viremic donors for heart transplant. J Heart Lung Transplant. 2022;41:37-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Logan C, Yumul I, Cepeda J, Pretorius V, Adler E, Aslam S, Martin NK. Cost-effectiveness of using hepatitis C viremic hearts for transplantation into HCV-negative recipients. Am J Transplant. 2021;21:657-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Bethea ED, Samur S, Kanwal F, Ayer T, Hur C, Roberts MS, Terrault N, Chung RT, Chhatwal J. Cost Effectiveness of Transplanting HCV-Infected Livers Into Uninfected Recipients With Preemptive Antiviral Therapy. Clin Gastroenterol Hepatol. 2019;17:739-747.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Kadatz M, Klarenbach S, Gill J, Gill JS. Cost-effectiveness of using kidneys from hepatitis C nucleic acid test-positive donors for transplantation in hepatitis C-negative recipients. Am J Transplant. 2018;18:2457-2464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Shelton BA, Sawinski D, Linas BP, Reese PP, Mustian M, Hungerpiller M, Reed RD, MacLennan PA, Locke JE. Population level outcomes and cost-effectiveness of hepatitis C treatment pre- vs postkidney transplantation. Am J Transplant. 2018;18:2483-2495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Jay CL, Skaro AI, Ladner DP, Wang E, Lyuksemburg V, Chang Y, Xu H, Talakokkla S, Parikh N, Holl JL, Hazen GB, Abecassis MM. Comparative effectiveness of donation after cardiac death versus donation after brain death liver transplantation: Recognizing who can benefit. Liver Transpl. 2012;18:630-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Javanbakht M, Mashayekhi A, Trevor M, Branagan-Harris M, Atkinson J. Cost-utility analysis of normothermic liver perfusion with the OrganOx metra compared to static cold storage in the United Kingdom. J Med Econ. 2020;23:1284-1292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | McMeekin N, Chrysos AE, Vale L, Fisher AJ. Incorporating ex-vivo lung perfusion into the UK adult lung transplant service: an economic evaluation and decision analytic model. BMC Health Serv Res. 2019;19:326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Northup PG, Abecassis MM, Englesbe MJ, Emond JC, Lee VD, Stukenborg GJ, Tong L, Berg CL; Adult-to-Adult Living Donor Liver Transplantation Cohort Study Group. Addition of adult-to-adult living donation to liver transplant programs improves survival but at an increased cost. Liver Transpl. 2009;15:148-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Sagmeister M, Mullhaupt B, Kadry Z, Kullak-Ublick GA, Clavien PA, Renner EL. Cost-effectiveness of cadaveric and living-donor liver transplantation. Transplantation. 2002;73:616-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Barnieh L, Gill JS, Klarenbach S, Manns BJ. The cost-effectiveness of using payment to increase living donor kidneys for transplantation. Clin J Am Soc Nephrol. 2013;8:2165-2173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Sarasin FP, Majno PE, Llovet JM, Bruix J, Mentha G, Hadengue A. Living donor liver transplantation for early hepatocellular carcinoma: A life-expectancy and cost-effectiveness perspective. Hepatology. 2001;33:1073-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 191] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Karnon J, Haghighi BM, Sajjad B, Yem S, Gamage A, Thorpe A. COST-UTILITY ANALYSIS OF PRIVATE CONTRACTING TO REDUCE PUBLIC WAITING TIMES FOR JOINT REPLACEMENT SURGERY. Int J Technol Assess Health Care. 2018;34:147-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Tuominen U, Sintonen H, Hirvonen J, Seitsalo S, Paavolainen P, Lehto M, Hietaniemi K, Blom M. Is longer waiting time for total knee replacement associated with health outcomes and medication costs? Randomized clinical trial. Value Health. 2010;13:998-1004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Mather RC 3rd, Hug KT, Orlando LA, Watters TS, Koenig L, Nunley RM, Bolognesi MP. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Hopkins RB, Tarride JE, Bowen J, Blackhouse G, O'Reilly D, Campbell K, Lim M, Goeree R. Cost-effectiveness of reducing wait times for cataract surgery in Ontario. Can J Ophthalmol. 2008;43:213-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Sach TH, Foss AJ, Gregson RM, Zaman A, Osborn F, Masud T, Harwood RH. Second-eye cataract surgery in elderly women: a cost-utility analysis conducted alongside a randomized controlled trial. Eye (Lond). 2010;24:276-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Borisenko O, Adam D, Funch-Jensen P, Ahmed AR, Zhang R, Colpan Z, Hedenbro J. Bariatric Surgery can Lead to Net Cost Savings to Health Care Systems: Results from a Comprehensive European Decision Analytic Model. Obes Surg. 2015;25:1559-1568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | van Egmond MMHT, Grutters JPC, Hannink G, van Heerbeek N, Rovers MM. Septoplasty versus non-surgical management for nasal obstruction in adults with a deviated septum: economic evaluation alongside a randomized controlled trial. BMC Med. 2020;18:101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Derrett S, Stokes EA, James M, Bartlett W, Bentley G. Cost and health status analysis after autologous chondrocyte implantation and mosaicplasty: a retrospective comparison. Int J Technol Assess Health Care. 2005;21:359-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Ribera A, Slof J, Ferreira-González I, Serra V, García-Del Blanco B, Cascant P, Andrea R, Falces C, Gutiérrez E, Del Valle-Fernández R, Morís-de laTassa C, Mota P, Oteo JF, Tornos P, García-Dorado D. The impact of waiting for intervention on costs and effectiveness: the case of transcatheter aortic valve replacement. Eur J Health Econ. 2018;19:945-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1676] [Cited by in F6Publishing: 1558] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 30. | Taylor TH, Jennings AM, Nightingale DA, Barber B, Leivers D, Styles M, Magner J. A study of anesthetic emergency work. I. The method of study and introduction to queuing theory. Br J Anaesth. 1969;41:70-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Aberg F, Mäklin S, Räsänen P, Roine RP, Sintonen H, Koivusalo AM, Höckerstedt K, Isoniemi H. Cost of a quality-adjusted life year in liver transplantation: the influence of the indication and the model for end-stage liver disease score. Liver Transpl. 2011;17:1333-1343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Bjørnelv GMW, Dueland S, Line PD, Joranger P, Fretland ÅA, Edwin B, Sørbye H, Aas E. Cost-effectiveness of liver transplantation in patients with colorectal metastases confined to the liver. Br J Surg. 2019;106:132-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Longworth L, Young T, Buxton MJ, Ratcliffe J, Neuberger J, Burroughs A, Bryan S; CELT Project Team. Midterm cost-effectiveness of the liver transplantation program of England and Wales for three disease groups. Liver Transpl. 2003;9:1295-1307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Volk ML, Vijan S, Marrero JA. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant. 2008;8:839-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 35. | Spolverato G, Vitale A, Ejaz A, Kim Y, Maithel SK, Cosgrove DP, Pawlik TM. The relative net health benefit of liver resection, ablation, and transplantation for early hepatocellular carcinoma. World J Surg. 2015;39:1474-1484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Webb AN, Lester ELW, Shapiro AMJ, Eurich DT, Bigam DL. Cost-utility analysis of normothermic machine perfusion compared to static cold storage in liver transplantation in the Canadian setting. Am J Transplant. 2022;22:541-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 37. | Kensinger CD, Dageforde LA, Moore DE. Can donors with high donor risk indices be used cost-effectively in liver transplantation in US Transplant Centers? Transpl Int. 2013;26:1063-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Dageforde LA, Feurer ID, Pinson CW, Moore DE. Is liver transplantation using organs donated after cardiac death cost-effective or does it decrease waitlist death by increasing recipient death? HPB (Oxford). 2013;15:182-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | McLean KA, Camilleri-Brennan J, Knight SR, Drake TM, Ots R, Shaw CA, Wigmore SJ, Harrison EM. Decision modeling in donation after circulatory death liver transplantation. Liver Transpl. 2017;23:594-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Schnitzler MA, Whiting JF, Brennan DC, Lin G, Chapman W, Lowell J, Boxerman S, Hardinger KL, Kalo Z. The expanded criteria donor dilemma in cadaveric renal transplantation. Transplantation. 2003;75:1940-1945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 41. | Dennen S, Díaz Espinosa O, Birch K, Cai J, Sung JC, Machado PGP, Shafrin J. Quantifying spillover benefits in value assessment: a case study of increased graft survival on the US kidney transplant waitlist. J Med Econ. 2021;24:918-928. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 42. | Kiberd BA, Tennankore KK, Vinson AJ. Comparing the Net Benefits of Adult Deceased Donor Kidney Transplantation for a Patient on the Preemptive Waiting List vs a Patient Receiving Dialysis. JAMA Netw Open. 2022;5:e2223325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 43. | Jassal SV, Krahn MD, Naglie G, Zaltzman JS, Roscoe JM, Cole EH, Redelmeier DA. Kidney transplantation in the elderly: a decision analysis. J Am Soc Nephrol. 2003;14:187-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Bieri U, Hübel K, Seeger H, Kulkarni GS, Sulser T, Hermanns T, Wettstein MS. Management of Active Surveillance-Eligible Prostate Cancer during Pretransplantation Workup of Patients with Kidney Failure: A Simulation Study. Clin J Am Soc Nephrol. 2020;15:822-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Yanev I, Gagnon M, Cheng MP, Paraskevas S, Kumar D, Dragomir A, Sapir-Pichhadze R. Kidney Transplantation in Times of Covid-19: Decision Analysis in the Canadian Context. Can J Kidney Health Dis. 2021;8:20543581211040332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Li B, Cairns JA, Johnson RJ, Watson CJE, Roderick P, Oniscu GC, Metcalfe W, Bradley JA, Tomson CR, Draper H, Forsythe JL, Dudley C, Ravanan R. Equity-Efficiency Trade-offs Associated With Alternative Approaches to Deceased Donor Kidney Allocation: A Patient-level Simulation. Transplantation. 2020;104:795-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Snyder RA, Moore DR, Moore DE. More donors or more delayed graft function? A cost-effectiveness analysis of DCD kidney transplantation. Clin Transplant. 2013;27:289-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Roels L, Kalo Z, Boesebeck D, Whiting J, Wight C. Cost-benefit approach in evaluating investment into donor action: the German case. Transpl Int. 2003;16:321-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 49. | Vinson AJ, Gala-Lopez B, Tennankore K, Kiberd B. The Use of Donation After Circulatory Death Organs for Simultaneous Liver-kidney Transplant: To DCD or Not to DCD? Transplantation. 2019;103:1159-1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Matas AJ, Schnitzler M. Payment for living donor (vendor) kidneys: a cost-effectiveness analysis. Am J Transplant. 2004;4:216-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Eckman MH, Woodle ES, Thakar CV, Alloway RR, Sherman KE. Cost-effectiveness of Using Kidneys From HCV-Viremic Donors for Transplantation Into HCV-Uninfected Recipients. Am J Kidney Dis. 2020;75:857-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 52. | Nguyen HD, Wong G, Howard K, Claas FH, Craig JC, Fidler S, D'Orsogna L, Chapman JR, Irish A, Ferrari P, Christiansen FT, Lim WH. Modeling the benefits and costs of integrating an acceptable HLA mismatch allocation model for highly sensitized patients. Transplantation. 2014;97:769-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Long EF, Swain GW, Mangi AA. Comparative survival and cost-effectiveness of advanced therapies for end-stage heart failure. Circ Heart Fail. 2014;7:470-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 54. | Vasiliadis HM, Collet JP, Penrod JR, Ferraro P, Poirier C. A cost-effectiveness and cost-utility study of lung transplantation. J Heart Lung Transplant. 2005;24:1275-1283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Mendonça L, Perelman J, Rodrigues V, Fragata J. Cost-effectiveness of lung transplantation and its evolution: the Portuguese case. Eur J Health Econ. 2014;15:767-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Anyanwu AC, McGuire A, Rogers CA, Murday AJ. An economic evaluation of lung transplantation. J Thorac Cardiovasc Surg. 2002;123:411-8; discussion 418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Feingold B, Webber SA, Bryce CL, Park SY, Tomko HE, West SC, Hart SA, Mahle WT, Smith KJ. Cost-effectiveness of pediatric heart transplantation across a positive crossmatch for high waitlist urgency candidates. Am J Transplant. 2015;15:2978-2985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Lopushinsky SR, Fowler RA, Kulkarni GS, Fecteau AH, Grant DR, Wales PW. The optimal timing of intestinal transplantation for children with intestinal failure: a Markov analysis. Ann Surg. 2007;246:1092-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |