Published online Apr 27, 2024. doi: 10.4240/wjgs.v16.i4.1109
Peer-review started: January 19, 2024
First decision: February 5, 2024
Revised: February 27, 2024
Accepted: March 26, 2024
Article in press: March 26, 2024
Published online: April 27, 2024
The incidence of gastric cancer has significantly increased in recent years. Surgical resection is the main treatment, but the method of digestive tract reconstruction after gastric cancer surgery remains controversial. In the current study, we sought to explore a reasonable method of digestive tract reconstruction and improve the quality of life and nutritional status of patients after surgery. To this end, we sta
To explore the application effect of DTR in total laparoscopic radical total gas
We collected the relevant data of 77 patients who underwent TLTG at the Fourth Hospital of Hebei Medical University from October 2021 to January 2023. Among them, 35 cases were treated with DTR, and the remaining 42 cases were treated with traditional RY. After 1:1 propensity score matching, the cases were grouped into 31 cases per group, with evenly distributed data. The clinical characteristics and short- and long-term clinical outcomes of the two groups were statistically analyzed.
The two groups showed no significant differences in basic data, intraoperative blood loss, number of lymph node dissections, first defecation time after operation, postoperative hospital stay, postoperative complications, and laboratory examination results on the 1st, 3rd, and 5th days after operation. The operation time of the DTR group was longer than that of the RY group [(307.58 ± 65.14) min vs (272.45 ± 62.09) min, P = 0.016], but the first intake of liquid food in the DTR group was shorter than that in the RY group [(4.45 ± 1.18) d vs (6.0 ± 5.18) d, P = 0.028]. The incidence of reflux heartburn (Visick grade) and postoperative gallbladder disease in the DTR group was lower than that in the RY group (P = 0.033 and P = 0.038). Although there was no significant difference in body weight, hemoglobin, prealbumin, and albumin between the two groups at 1,3 and 6 months after surgery, the diet of pa
The clinical effect of DTR in TLTG is better than that of RY, indicating that it is a more valuable digestive tract reconstruction method in laparoscopic gastric cancer surgery.
Core Tip: We statistically analyzed the clinical results of patients with gastric cancer who underwent jejunal interposition double-tract reconstruction (DTR) and esophageal jejunum Roux-en-Y reconstruction (RY). Finally, it was found that compared to RY, DTR can improve postoperative life treatment, improve postoperative nutritional status, and reduce the incidence of gallbladder disease. It can also provide a duodenal pathway for endoscopic retrograde cholangio pancreatography, representing a better digestive tract reconstruction method than traditional Roux-en-Y esophagojejunostomy.
- Citation: Dong TX, Wang D, Zhao Q, Zhang ZD, Zhao XF, Tan BB, Liu Y, Liu QW, Yang PG, Ding PA, Zheng T, Li Y, Liu ZJ. Comparative analysis of two digestive tract reconstruction methods in total laparoscopic radical total gastrectomy. World J Gastrointest Surg 2024; 16(4): 1109-1120
- URL: https://www.wjgnet.com/1948-9366/full/v16/i4/1109.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i4.1109
Gastric cancer is the fourth most common malignant tumor worldwide and is one of the leading causes of cancer-related death[1]. Surgical resection of the entire tumor and complete lymph node dissection are the main treatments for gastric cancer[2]. With the development of laparoscope technology and equipment, radical laparoscopic surgery for gastric cancer has been widely performed in clinical practice[3]. Currently, the incidence of proximal gastric cancer and eso
Esophageal jejunum Roux-en-Y reconstruction (RY) is the main method of digestive tract reconstruction after laparoscopic total gastrectomy because it is simple, safe, and has an acceptable probability of serious complications after sur
At that time, the operation of the DTR was too complicated and the operation time was too long and technically challenging. Therefore, there have been few reports on the completion of DTR under total laparoscopy by domestic and foreign researchers. However, with the iterative update of anastomosis instruments and barbed sutures, a good platform has been built for completing DTR under total laparoscopy. In the process of laparoscopic reconstruction of digestive tract anastomosis, the volume of the circular stapler is larger than that of the linear cutting stapler; therefore, it is necessary to close and reconstruct the pneumoperitoneum and complete the anastomosis repeatedly with the help of the abdominal small incision, which increases the complexity of the operation to some extent. In our center, we use a linear cutting stapler to complete the laparoscopic DTR, avoiding the cumbersome steps of circular stapler anvil and operating rod placement, duodenal purse-string suture, and posterior wall reinforcement suture, thereby reducing the operation time and improving the safety of the operation[9]. The purpose of this study was to explore the application effect of DTR in total laparoscopic radical total gastrectomy (TLTG) and to evaluate its safety and effectiveness.
We retrospectively analyzed the data of 77 patients who underwent TLTG at the Fourth Hospital of Hebei Medical University from October 2021 to January 2023, comprising 35 cases undergoing DTR and 42 cases of RY. After propensity score matching (PSM), the patients were grouped into 31 cases per group. The inclusion criteria were as follows: (1) Pa
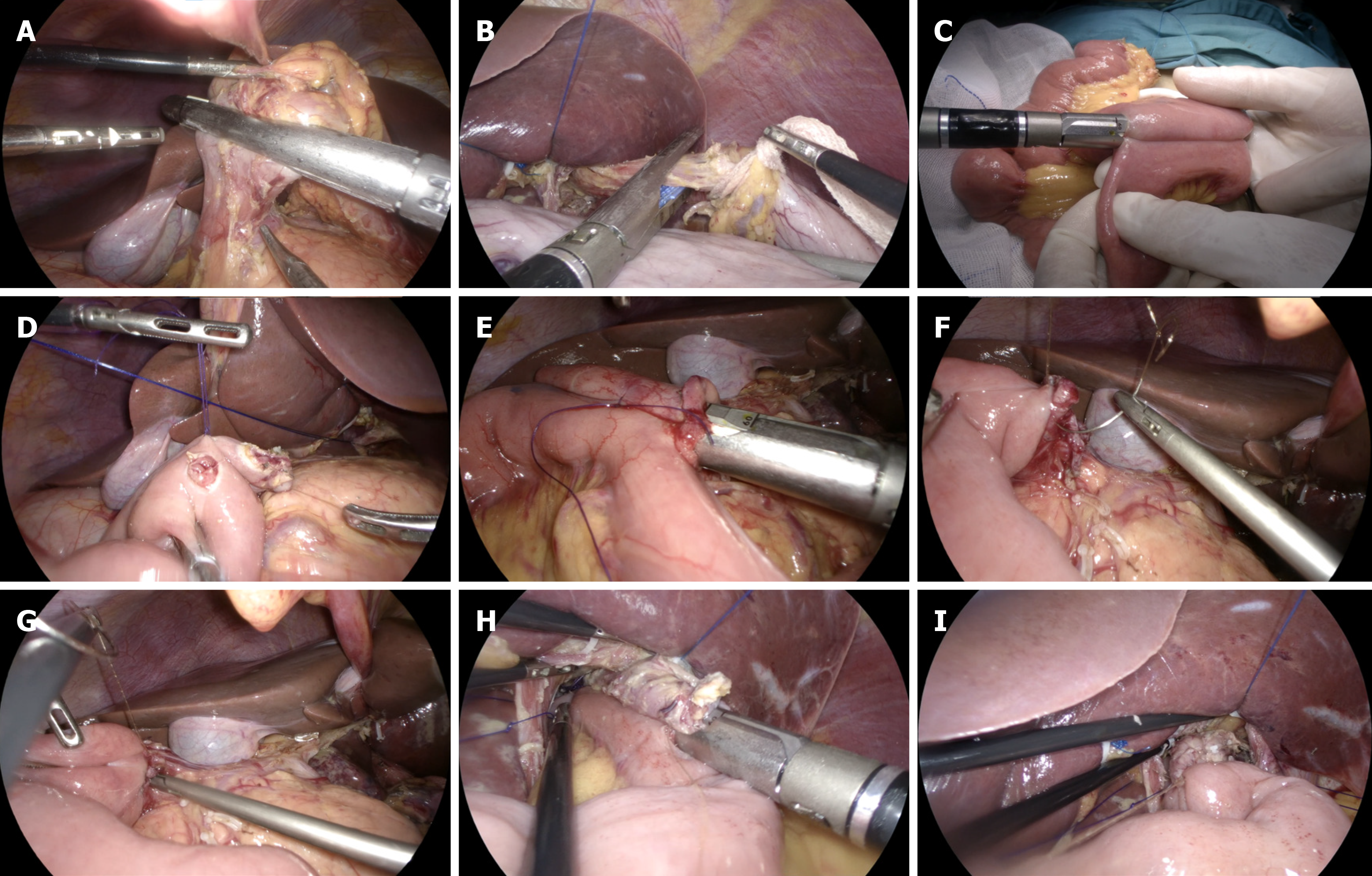
DTR: (1) D2 Lymph node dissection and total gastrectomy were performed under laparoscopy. The pneumoperitoneum was closed, and the specimen was removed from the supraumbilical median incision (approximately 4 cm in length). After confirming the safety of the upper residual distance of the specimen, it was sent for histopathological examination. The upper jejunum was lifted outside the abdominal cavity. An ultrasonic scalpel was used to open the jejunal mesenteric avascular area at approximately 20 cm from the Treitz ligament, and the vascular arch was ligated and cut off. The jejunum was then severed using a linear cutting stapler. Jejunal side-to-side anastomosis was performed at a distance of 60 cm from the distal jejunum blind loop, and the mesenteric hiatus was closed (Figure 1A-C); (2) After reconstructing the pneumoperitoneum, it was confirmed that the distal jejunum (jejunal food loop) could be easily lifted to the site of the esophageal stump. The area 35 cm away from the jejunal blind loop was fixed with the descending duodenum using an absorbable suture. Then, the ultrasonic scalpel was used to open the above two positions (Figure 1D); (3) A linear cutting stapler (white nail) with a length of 60 or 45 mm was inserted into the abdominal cavity through the left upper Trocar orifice. The two arms of the linear cutting stapler were placed at the above two openings to complete the side-to-side anastomosis of the duodenum–jejunum (the width of the anastomosis was 40–45 mm) (Figure 1E); (4) The duode
RY: (1) D2 Lymph node dissection and total gastrectomy were performed under laparoscopy. The pneumoperitoneum was closed and the specimen was removed from the supraumbilical median incision (approximately 4 cm in length). After confirming the safety of the upper residual distance of the specimen, it was sent for histopathological examination. The upper jejunum was lifted outside the abdominal cavity. An ultrasonic scalpel was used to open the jejunal mesenteric avascular area at approximately 20 cm from the Treitz ligament, and the vascular arch was ligated and cut off. The je
According to the medical and anesthesia records, we obtained the sex, age, body mass index (BMI), tumor location, pathological stage, tumor size, operation time, intraoperative blood loss, number of lymph node dissections, postope
Statistical analyses were performed using SPSS 26.0. A two-sample Student’s t-test or rank sum test was used to compare continuous variables, and the χ2 test or Fisher’s exact test was performed for categorical variables. The rank sum test was used for grade data. P values < 0.05 were considered statistically significant. All observed factors were subjected to PSM, and the matching caliper value was 0.2. Continuous variables are represented as the mean ± SD, and categorical variables are represented as n (%).
A total of 77 patients with gastric cancer were included in our study, including 60 males and 17 females; the average age of the patients was (58.49 ± 11.10) years, ranging from 26 to 77 years; and the average BMI was (24.79 ± 3.54). In terms of the tumor location, 44 cases were in the upper part of the gastric body, 15 cases were in the middle part of the gastric body, and 18 cases were in the lower part of the gastric body. Regrading postoperative pathological staging, 20 cases were stage I, 21 cases were stage II, and 36 cases were stage III. The mean longest tumor diameter was (4.40 ± 2.33) cm. Additionally, there were 35 cases of DTR and 42 cases of RY.
The following six covariates were selected for PSM: Sex, age, BMI, tumor location, postoperative pathological stage, and longest tumor diameter. After 1:1 PSM, 62 patients were finally included in the study (Table 1), with no significant di
| Variables | Before PSM | After PSM | ||||
| DTR (n = 35) | RY (n = 42) | P value | DTR (n = 31) | RY (n = 31) | P value | |
| Age (yr) | 0.582 | 1 | ||||
| ≤ 60 | 20 (57.1) | 19 (45.2) | 16 (51.6) | 16 (51.6) | ||
| > 60 | 15 (42.9) | 23 (54.8) | 15 (48.4) | 15 (48.4) | ||
| Sex | 0.989 | 1 | ||||
| Female | 8 (22.9) | 9 (21.4) | 7 (22.6) | 7 (22.6) | ||
| Male | 27 (77.1) | 33 (78.6) | 24 (77.4) | 24 (77.4) | ||
| BMI | 0.932 | 0.594 | ||||
| 18.4-24.2 | 16 (45.7) | 21 (50.0) | 16 (51.6) | 12 (38.7) | ||
| 24.3-34.67 | 19 (54.3) | 21 (50.0) | 15 (48.4) | 19 (61.3) | ||
| Tumor location | 1 | 0.968 | ||||
| Upper | 20 (57.1) | 24 (57.1) | 18 (58.1) | 17 (54.8) | ||
| Middle/lower | 15 (42.9) | 18 (42.9) | 13 (41.9) | 14 (45.2) | ||
| TNM stage | 0.481 | 0.878 | ||||
| I/II | 16 (45.7) | 25 (59.5) | 16 (51.6) | 18 (58.1) | ||
| III | 19 (54.3) | 17 (40.5) | 15 (48.4) | 13 (41.9) | ||
| Longest tumor diameter (cm) | 0.707 | 0.968 | ||||
| 0.8-3.9 | 15 (42.9) | 22 (52.4) | 15 (48.4) | 16 (51.6) | ||
| 4.0-14.0 | 20 (57.1) | 20 (47.6) | 16 (51.6) | 15 (48.4) | ||
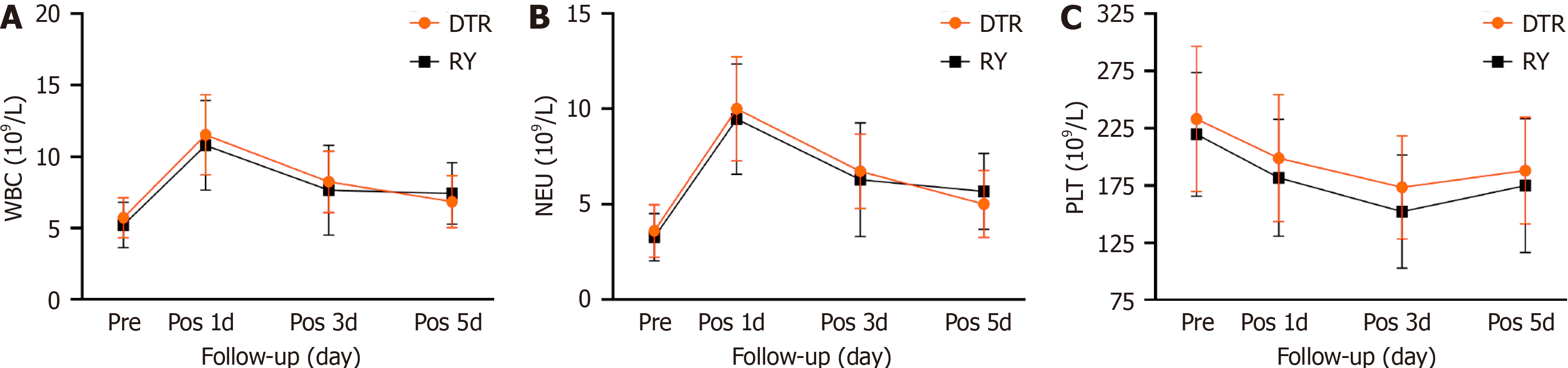
The two groups showed no significant difference in intraoperative blood loss, number of lymph node dissections, postoperative first defecation time, and postoperative hospital stay. The operation time of the DTR group was longer than that of the RY group [(307.58 ± 65.14) min vs (272.45 ± 62.09) min, P = 0.016]. The first intake of liquid food in the DTR group was shorter than that in the RY group [(4.45 ± 1.18) d vs (6.0 ± 5.18) d, P = 0.028]. In terms of surgical complications, there was no significant statistical difference between the two groups. There were 21 cases of type II complications in the DTR group, without abdominal infection or anastomotic-related complications. There were 25 cases of type II complications and two cases of type III complications in the RY group. In the RY group, one patient had abdominal infection and anastomotic bleeding and one patient had esophagojejunal anastomotic leakage. The two patients were cured and discharged after conservative treatment. There were no cases of death between the two groups of patients, and they were discharged smoothly (Table 2). Additionally, there was no significant difference in the levels of white blood cells, neu
| DTR (n = 31) | RY (n = 31) | P value | |
| Operation time (min) | 307.58 ± 65.14 | 272.45 ± 62.09 | 0.016a |
| Blood loss during operation (mL) | 47.42 ± 29.77 | 47.58 ± 29.21 | 0.971 |
| Number of lymph node dissection | 45.16 ± 15.10 | 47.97 ± 16.84 | 0.512 |
| The first postoperative exhaust time (d) | 3.23 ± 0.72 | 3.06 ± 1.18 | 0.158 |
| The first time to eat liquid food after operation (d) | 4.45 ± 1.18 | 6.0 ± 5.18 | 0.028a |
| Hospital stay after operation (d) | 7.71 ± 2.15 | 8.87 ± 5.49 | 0.65 |
| Operative complications | 0.079 | ||
| Grade II | |||
| Pneumonia | 17 | 22 | |
| Lower limb thrombosis | 11 | 14 | |
| Grade III | |||
| Abdominal infection | 0 | 2 | |
| Anastomotic bleeding | 0 | 1 | |
| Esophageal jejunum anastomotic fistula | 0 | 1 | |
| White blood cells (109/L) | |||
| Preoperative | 5.73 ± 1.41 | 5.22 ± 1.59 | 0.19 |
| The first day after surgery | 11.51 ± 2.81 | 10.79 ± 3.14 | 0.344 |
| The third day after surgery | 8.24 ± 2.15 | 7.65 ± 3.13 | 0.39 |
| The fifth day after surgery | 6.86 ± 1.82 | 7.43 ± 2.15 | 0.265 |
| Neutrophils (109/L) | |||
| Preoperative | 3.61 ± 1.38 | 3.28 ± 1.25 | 0.32 |
| The first day after surgery | 10.01 ± 2.73 | 9.47 ± 2.89 | 0.449 |
| The third day after surgery | 6.74 ± 1.95 | 6.29 ± 2.97 | 0.486 |
| The fifth day after surgery | 5.02 ± 1.75 | 5.68 ± 1.99 | 0.167 |
| Platelets (109/L) | |||
| Preoperative | 232.97 ± 63.29 | 219.65 ± 54.07 | 0.376 |
| The first day after surgery | 198.77 ± 55.44 | 181.77 ± 51.13 | 0.214 |
| The third day after surgery | 173.45 ± 45.10 | 152.19 ± 49.45 | 0.082 |
| The fifth day after surgery | 187.94 ± 46.52 | 174.87 ± 58.41 | 0.334 |
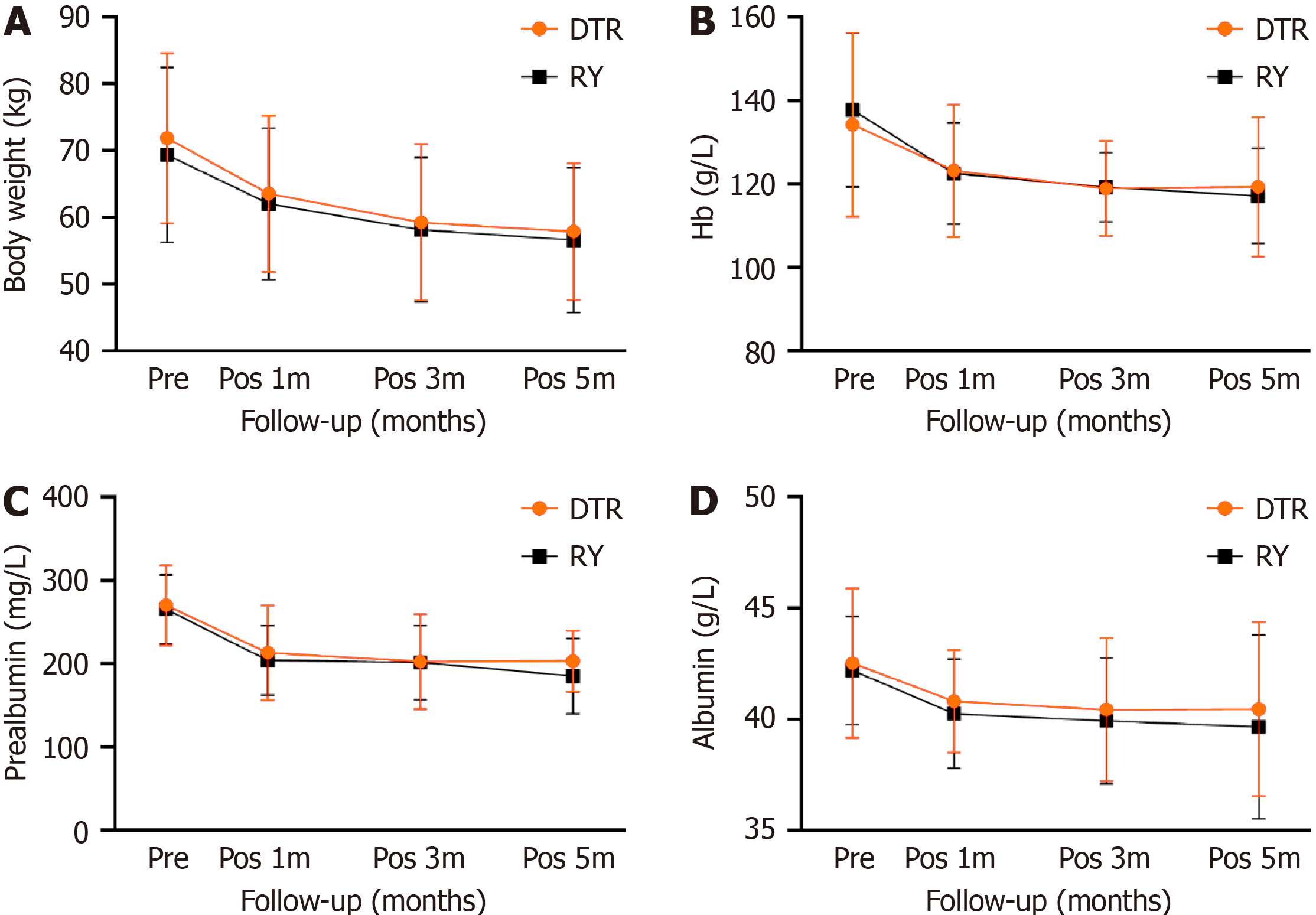
The nutritional status between the two groups was compared by assessing body weight, diet recovery, and hemoglobin, prealbumin, and albumin levels. There was no significant difference in body weight, hemoglobin, prealbumin, and albumin levels between the two groups before surgery. At 1, 3, and 6 months after surgery, the body weight, hemoglobin, albumin, and prealbumin levels of the two groups decreased, with no significant difference between the two groups (Figure 3). The ratio of food intake in the DTR group was higher than that in the RY group at 6 months after surgery [(89.61 ± 14.75)% vs (80.06 ± 17.60)%, P = 0.031]. According to the CT results of the two groups of patients at 6 months after the operation, the incidence of gallbladder disease (including cholecystitis, gallstones, bile salt deposition) was determined. The incidence of gallbladder disease in the DTR group was 6.5%. The probability of gallbladder disease in the RY group was 25.8%, with a significant statistical difference (P = 0.038). During the 6-month follow-up, the post
| DTR (n = 31) | RY (n = 31) | P value | |
| Food intake ratio (%) | 89.61 ± 14.75 | 80.06 ± 17.60 | 0.031a |
| Gallbladder disease, n (%) | 0.038a | ||
| Positive | 2 (6.5) | 8 (25.8) | |
| Negative | 29 (93.5) | 23 (74.2) | |
| Visick grade, n (%) | 0.033a | ||
| I | 18 (61.3) | 11 (35.4) | |
| II | 12 (35.5) | 14 (45.2) | |
| III | 1 (3.2) | 6 (19.4) | |
| Weight (kg) | |||
| Preoperative | 71.86 ± 12.77 | 69.34 ± 13.14 | 0.447 |
| Pos 1m | 63.5 ± 11.73 | 62.03 ± 11.34 | 0.618 |
| Pos 3m | 59.24 ± 11.74 | 58.15 ± 10.84 | 0.704 |
| Pos 6m | 57.85 ± 10.25 | 56.55 ± 10.90 | 0.631 |
| Hemoglobin (g/L) | |||
| Preoperative | 134.22 ± 22.05 | 137.77 ± 18.49 | 0.495 |
| Pos 1m | 123.16 ± 15.84 | 122.48 ± 12.16 | 0.849 |
| Pos 3m | 118.97 ± 11.39 | 119.21 ± 8.39 | 0.925 |
| Pos 6m | 119.32 ± 16.68 | 117.20 ± 11.45 | 0.561 |
| Prealbumin (mg/L) | |||
| Preoperative | 270.09 ± 48.0 | 265.52 ± 41.41 | 0.689 |
| Pos 1m | 213.17 ± 56.55 | 204.18 ± 41.68 | 0.479 |
| Pos 3m | 202.37 ± 57.15 | 201.34 ± 44.37 | 0.937 |
| Pos 6m | 203.25 ± 36.66 | 185.12 ± 45.44 | 0.089 |
| Albumin (g/L) | |||
| Preoperative | 42.51 ± 3.35 | 42.20 ± 2.44 | 0.672 |
| Pos 1m | 40.80 ± 2.31 | 40.25 ± 2.45 | 0.368 |
| Pos 3m | 40.43 ± 3.21 | 39.93 ± 2.83 | 0.521 |
| Pos 6m | 40.45 ± 3.91 | 39.66 ± 4.12 | 0.44 |
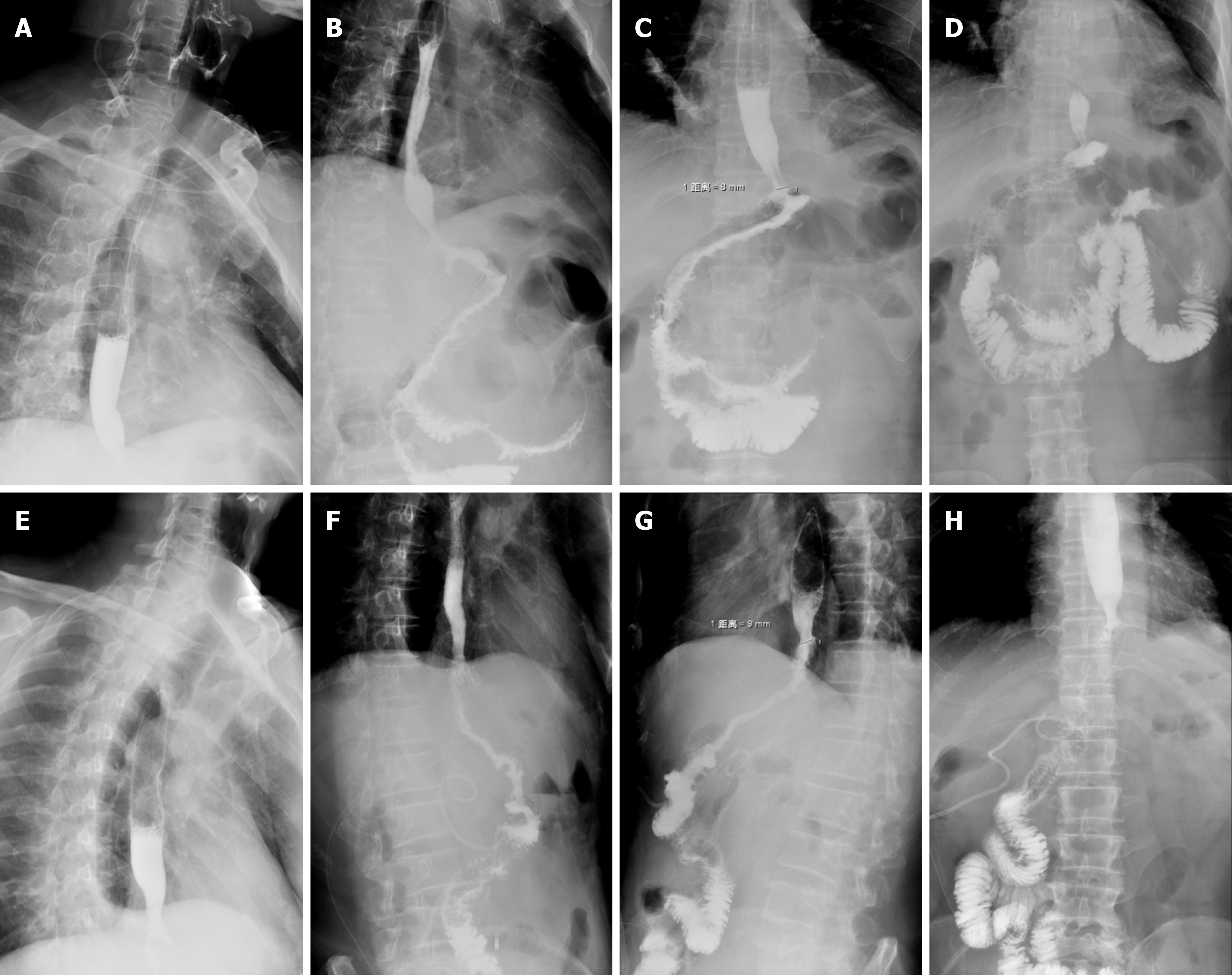
The two groups of patients completed upper gastrointestinal angiography approximately 1 wk after surgery. Angio
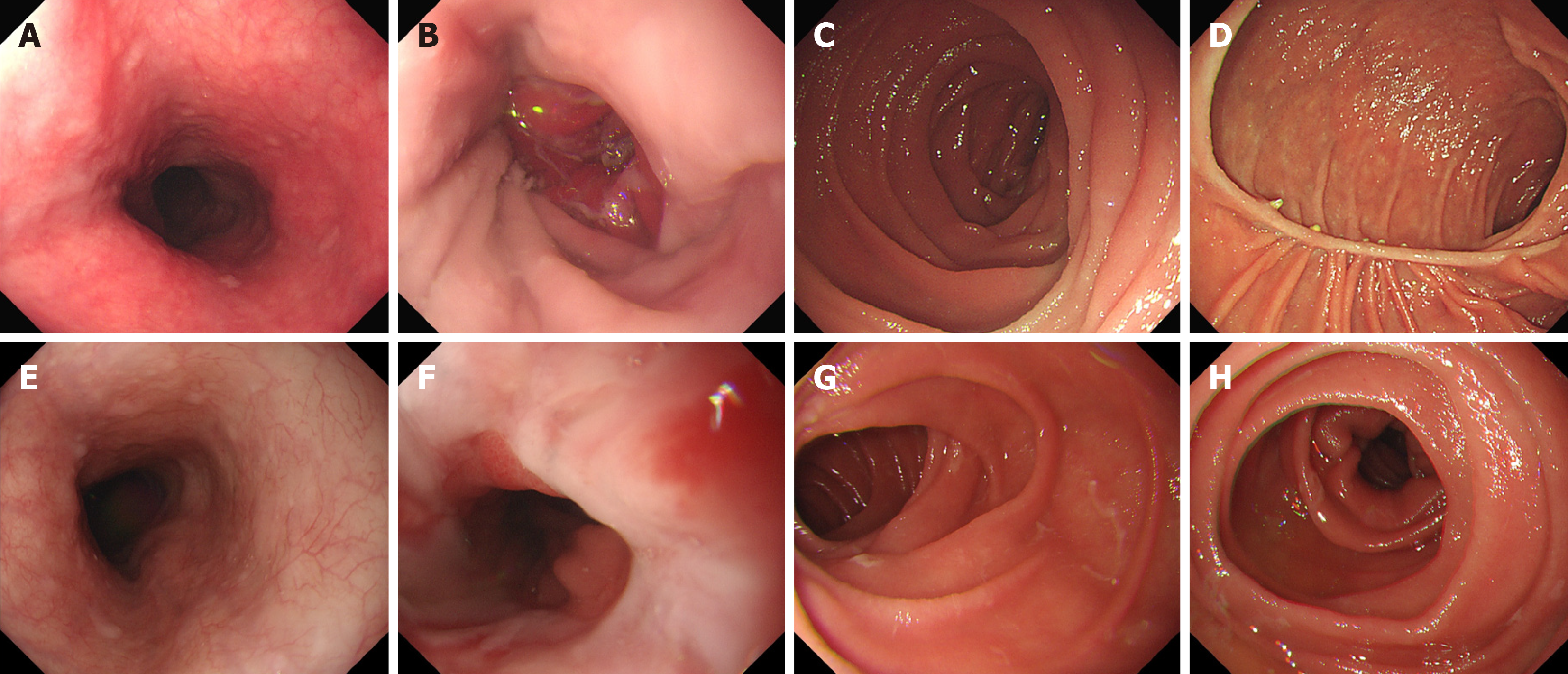
The patients completed gastroscopy 6 months after the operation. For the patients in the DTR group, it was possible to see the duodenal jejunum anastomosis under gastroscopy, and the width of the anastomosis was approximately 45 mm. No anastomotic ulcer, stenosis, bleeding, or canceration were found in the two groups under gastroscopy. The gastroscopy results of the DTR and RY groups are shown in Figure 5.
Total laparoscopic total gastrectomy has been increasingly used since Uyama et al[11] first reported the study of total laparoscopic total gastrectomy in 1999. Compared to open surgery, laparoscopic gastric cancer surgery has the ad
The results showed no significant difference in intraoperative blood loss, number of lymph node dissections, first postoperative defecation time, postoperative hospital stay, surgical complications, and postoperative laboratory test results between the two groups of patients. The operation time of the DTR group was longer than that of the RY group [(307.58 ± 65.14) min vs (272.45 ± 62.09) min, P = 0.016], but the first intake of liquid food in the DTR group was shorter than that in the RY group [(4.45 ± 1.18) d vs (6.0 ± 5.18) d, P = 0.028]. These results confirm the safety and feasibility of DTR, which has the same satisfactory postoperative recovery effect as RY. In general, the risk of DTR is the increase in the number of anastomotic stomas, which increases the probability of postoperative anastomotic complications[8,17]. How
Although there was no significant difference in body weight, hemoglobin, prealbumin, and albumin between the two groups at 1, 3, and 6 months after operation, the hemoglobin, prealbumin, and albumin levels in the DTR group were higher than those in the RY group at 1, 3, and 6 months after the operation. During the 6-month follow-up, the food intake ratio of the DTR group was also significantly better than that of the RY group (P = 0.031). Some studies have suggested that DTR has better food reserve function after gastrectomy, which may influence food intake, which is con
In this study, the symptoms of acid reflux and heartburn in the DTR group were significantly lower than those in the RY group (P = 0.033). Previous reports have confirmed that RY can effectively prevent reflux esophagitis[19,20], but some patients still exhibit severe reflux heartburn symptoms after surgery. In our follow-up of 31 patients with RY, 20 (64.5%) had reflux heartburn symptoms, including six (19.3%) that were Visick grade III, which is similar to the results of Chen et al’s study[21]. Reflux heartburn symptoms not only affect the quality of life of patients after surgery but also affect the patient’s food intake, indirectly leading to a decline in patient weight[7]. In the DTR group, alkaline digestive juice can flow into the jejunum food branch when reflux occurs due to the presence of dual pathway. Moreover, the length of our interposed small intestine is approximately 35 cm, which will also effectively reduce the occurrence of this complication[14,22].
The incidence of gallbladder disease in patients after RY is generally higher than that in other patients because food does not pass through the duodenum and lacks the stimulation of food to bile secretion[23]. In our study, the incidence of postoperative gallbladder disease in patients with DTR was 6.5%, while the probability of gallbladder disease in the RY group was 25.8%. Additionally, there was a significant difference between the two groups, which was consistent with previous research results[23]. Once gallbladder disease occurs in patients undergoing RY, due to the exclusion of the duodenal pathway, it will be difficult for the endoscope to enter the biliary system for examination or treatment; at this point, only invasive operation or surgery can be used for treatment[22,24]. Therefore, when performing digestive tract reconstruction, it is important to consider the needs of endoscopy, and DTR meets this requirement[8]. Some studies have shown that the preservation of the duodenal pathway also has a positive effect on the long-term prognosis of patients[19,25-27].
In summary, although DTR has a slight increase in operation time, this does not impact the patient’s postoperative recovery and the incidence of anastomotic-related complications. It can also improve the quality of life of patients after surgery, improve postoperative nutritional status, and reduce the incidence of gallbladder disease. Simultaneously, it can also provide a duodenal pathway for endoscopic retrograde cholangio pancreatography. Although we demonstrate that DTR is a better digestive tract reconstruction method than traditional RY, the limitations of this study include the small sample size, the short postoperative follow-up time, and the lack of further analysis of anemia indicators such as vitamin B12 and ferritin. In addition, prospective studies are still needed for comparative analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Isobe T, Japan S-Editor: Yan JP L-Editor: A P-Editor: Xu ZH
| 1. | Stegniy KV, Maslyantsev EV, Goncharuk RA, Krekoten AA, Kulakova TA, Dvoinikova ER. Double-tract reconstruction for oesofagocardial gastric cancer: A systematic review. Ann Med Surg (Lond). 2021;67:102496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Yun L, Zhiwei J, Junsheng P, Xiaobin W, Cancan X, Jieshou L. Comparison of Functional Outcomes between Functional Jejunal Interposition and Conventional Roux-en-Y Esophagojejunostomy after Total Gastrectomy for Gastric Cancer. Dig Surg. 2020;37:240-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Chen Y, Zheng T, Chen Y, Zheng Y, Tan S, Liu S, Zhou Y, Lin X, Chen W, Mi Y, Lin S, Yang C, Li W. Totally laparoscopic total gastrectomy with Uncut Roux-en-Y for gastric cancer may improve prognosis: A propensity score matching comparative study. Front Oncol. 2022;12:1086966. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 4. | Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J, Du X, Huang H, Hu J, Li G, Yu P, Li Y, Suo J, Zhao N, Zhang W, Li H, He H, Sun Y; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: The CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol. 2020;6:1590-1597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 104] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 5. | Umemura A, Koeda K, Sasaki A, Fujiwara H, Kimura Y, Iwaya T, Akiyama Y, Wakabayashi G. Totally laparoscopic total gastrectomy for gastric cancer: literature review and comparison of the procedure of esophagojejunostomy. Asian J Surg. 2015;38:102-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Zhao Y, Zhang J, Yang D, Tang Z, Wang Q. Feasibility of laparoscopic total gastrectomy for advanced Siewert type Ⅱ and type Ⅲ esophagogastric junction carcinoma: A propensity score-matched case-control study. Asian J Surg. 2019;42:805-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Ikeguchi M, Kuroda H, Saito H, Tatebe S, Wakatsuki T. A new pouch reconstruction method after total gastrectomy (pouch-double tract method) improved the postoperative quality of life of patients with gastric cancer. Langenbecks Arch Surg. 2011;396:777-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Hong J, Wang SY, Hao HK. A Comparative Study of Double-Tract Reconstruction and Roux-en-Y After Gastrectomy for Gastric Cancer. Surg Laparosc Endosc Percutan Tech. 2019;29:82-89. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Kim JJ, Song KY, Chin HM, Kim W, Jeon HM, Park CH, Park SM. Totally laparoscopic gastrectomy with various types of intracorporeal anastomosis using laparoscopic linear staplers: preliminary experience. Surg Endosc. 2008;22:436-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Chen Z, Wang D, Zhao Q, Yang P, Ding P, Fan H, Dong T, Liu Z, Yang X, Ren L, Li Y. A case series of 10 patients undergone linear cutter/stapler guiding device-led overlapped esophagojejunostomy: a preliminary study. J Gastrointest Oncol. 2023;14:617-625. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 11. | Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer. 1999;2:230-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 186] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Zhao S, Zheng K, Zheng JC, Hou TT, Wang ZN, Xu HM, Jiang CG. Comparison of totally laparoscopic total gastrectomy and laparoscopic-assisted total gastrectomy: A systematic review and meta-analysis. Int J Surg. 2019;68:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Syn NL, Wee I, Shabbir A, Kim G, So JB. Pouch Versus No Pouch Following Total Gastrectomy: Meta-analysis of Randomized and Non-randomized Studies. Ann Surg. 2019;269:1041-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Li Z, Dong J, Huang Q, Zhang W, Tao K. Comparison of three digestive tract reconstruction methods for the treatment of Siewert II and III adenocarcinoma of esophagogastric junction: a prospective, randomized controlled study. World J Surg Oncol. 2019;17:209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Burm AG, van Kleef JW, Gladines MP, van Duinen M, Spierdijk J. Spinal anesthesia with hyperbaric lidocaine and bupivacaine: effects of epinephrine on the plasma concentration profiles. Anesth Analg. 1987;66:1104-1108. [PubMed] [Cited in This Article: ] |
| 16. | Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Completely laparoscopic proximal gastrectomy with jejunal interposition and lymphadenectomy. J Am Coll Surg. 2000;191:114-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Ji X, Jin C, Ji K, Zhang J, Wu X, Jia Z, Bu Z, Ji J. Double Tract Reconstruction Reduces Reflux Esophagitis and Improves Quality of Life after Radical Proximal Gastrectomy for Patients with Upper Gastric or Esophagogastric Adenocarcinoma. Cancer Res Treat. 2021;53:784-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Liu YH, Meng R, Zhu B, Zhan QQ, Yang X, Ding GY, Jia CL, Xu WG. A meta-analysis of the efficacy of Roux-en-Y anastomosis and jejunal interposition after total gastrectomy. World J Surg Oncol. 2023;21:136. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 19. | Iwahashi M, Nakamori M, Nakamura M, Naka T, Ojima T, Iida T, Katsuda M, Ueda K, Yamaue H. Evaluation of double tract reconstruction after total gastrectomy in patients with gastric cancer: prospective randomized controlled trial. World J Surg. 2009;33:1882-1888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Fan KX, Xu ZF, Wang MR, Li DT, Yang XS, Guo J. Outcomes for jejunal interposition reconstruction compared with Roux-en-Y anastomosis: A meta-analysis. World J Gastroenterol. 2015;21:3093-3099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Chen W, Jiang X, Huang H, Ding Z, Li C. Jejunal pouch reconstruction after total gastrectomy is associated with better short-term absorption capacity and quality of life in early-stage gastric cancer patients. BMC Surg. 2018;18:63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Xiao JW, Liu ZL, Ye PC, Luo YJ, Fu ZM, Zou Q, Wei SJ. Clinical comparison of antrum-preserving double tract reconstruction vs roux-en-Y reconstruction after gastrectomy for Siewert types II and III adenocarcinoma of the esophagogastric junction. World J Gastroenterol. 2015;21:9999-10007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 23. | Ikegame K, Hikage M, Fujiya K, Kamiya S, Tanizawa Y, Bando E, Notsu A, Terashima M. The Effect of Minimally Invasive Gastrectomy for Gastric Cancer on Postoperative Gallstone Formation. World J Surg. 2021;45:3378-3385. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 24. | Namikawa T, Kitagawa H, Okabayashi T, Sugimoto T, Kobayashi M, Hanazaki K. Double tract reconstruction after distal gastrectomy for gastric cancer is effective in reducing reflux esophagitis and remnant gastritis with duodenal passage preservation. Langenbecks Arch Surg. 2011;396:769-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Ishigami S, Natsugoe S, Hokita S, Aoki T, Kashiwagi H, Hirakawa K, Sawada T, Yamamura Y, Itoh S, Hirata K, Ohta K, Mafune K, Nakane Y, Kanda T, Furukawa H, Sasaki I, Kubota T, Kitajima M, Aikou T. Postoperative long-term evaluation of interposition reconstruction compared with Roux-en-Y after total gastrectomy in gastric cancer: prospective randomized controlled trial. Am J Surg. 2011;202:247-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Ogoshi K, Okamoto Y, Nabeshima K, Morita M, Nakamura K, Iwata K, Soeda J, Kondoh Y, Makuuchi H. Focus on the conditions of resection and reconstruction in gastric cancer. What extent of resection and what kind of reconstruction provide the best outcomes for gastric cancer patients? Digestion. 2005;71:213-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Hu Y, Zaydfudim VM. Quality of Life After Curative Resection for Gastric Cancer: Survey Metrics and Implications of Surgical Technique. J Surg Res. 2020;251:168-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |