Copyright
©The Author(s) 2024.
World J Gastrointest Surg. Mar 27, 2024; 16(3): 882-892
Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.882
Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.882
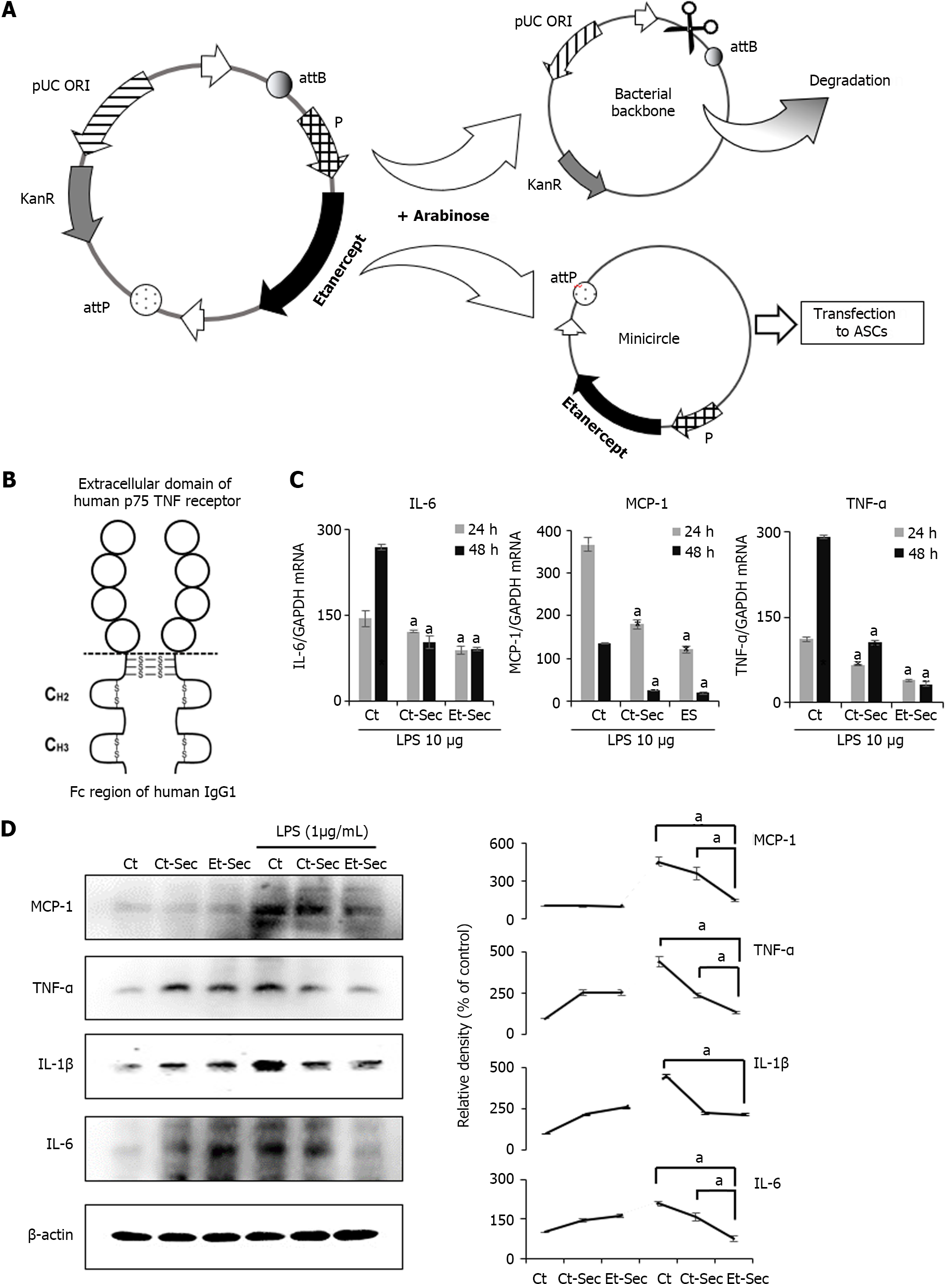
Figure 1 Generation of etanercept-secretome and assessment of its in vitro anti-inflammatory effects.
A: Schematic representation of the mini-circle plasmid encoding etanercept derived from the parental plasmid through arabinose treatment; B: Etanercept Structure. Etanercept, a protein-based drug with tumor necreosis factor (TNF)-α receptor and IgG1 Fc domains, effectively binds and neutralizes TNF-α, a critical inflammatory cytokine; C: Real-time polymerase chain reaction of inflammatory markers. In the etanercept-secretome (Et-Sec) group, mRNA expression levels of TNF-α and interleukin (IL)-6 were significantly lower compared to other groups [control group and control-secretome (Ct-Sec) group] at 24 and 48 h, after lipopolysaccharide (LPS) treatment (P < 0.05); D: Western blot analysis of inflammatory markers. Et-Sec treatment led to a significant reduction in Monocyte Chemoattractant Protein-1, TNF-α, and IL-6 expression levels when compared to the Ct-Sec treatment in LPS-induced inflammation in CCD-18Co colon normal cells (P < 0.05). Relative densities of individual markers had been quantified using Image J software and then were normalized to that of β-actin in each group. Values are presented as mean ± SD of three independent experiments. aP < 0.05. Et-Sec: Etanercept-secretome; Ct-Sec: Control-secretome; LPS: Lipopolysaccharide; TNF: Tumor necreosis factor; MCP-1: Monocyte chemoattactant protein-1; IL: Interleukin; IBD: Inflammatory bowel disease; DSS-treated group: Dextran sulfate sodium-treated group; ASCs: Adipose-derived stem cells; Ct: Control group.
- Citation: Kim SJ, Kim OH, Hong HE, Ju JH, Lee DS. Etanercept-synthesizing adipose-derived stem cell secretome: A promising therapeutic option for inflammatory bowel disease. World J Gastrointest Surg 2024; 16(3): 882-892
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/882.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.882