Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.882
Peer-review started: November 9, 2023
First decision: December 8, 2023
Revised: January 3, 2024
Accepted: February 3, 2024
Article in press: February 3, 2024
Published online: March 27, 2024
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the gastrointestinal tract, with tumor necrosis factor (TNF)-α playing a key role in its pathogenesis. Etanercept, a decoy receptor for TNF, is used to treat inflammatory conditions. The secretome derived from adipose-derived stem cells (ASCs) has anti-inflammatory effects, making it a promising therapeutic option for IBD.
To investigate the anti-inflammatory effects of the secretome obtained from ASCs synthesizing etanercept on colon cells and in a dextran sulfate sodium (DSS)-induced IBD mouse model.
ASCs were transfected with etanercept-encoding mini-circle plasmids to create etanercept-producing cells. The secretory material from these cells was then tested for anti-inflammatory effects both in vitro and in a DSS-induced IBD mouse model.
This study revealed promising results indicating that the group treated with the secretome derived from etanercept-synthesizing ASCs [Etanercept-Secretome (Et-Sec) group] had significantly lower expression levels of inflammatory mediators, such as interleukin-6, Monocyte Chemoattractant Protein-1, and TNF-α, when compared to the control secretome (Ct-Sec). Moreover, the Et-Sec group exhibited a marked therapeutic effect in terms of preserving the architecture of intestinal tissue compared to the Ct-Sec.
These results suggest that the secretome derived from ASCs that synthesize etanercept has potential as a therapeutic agent for the treatment of IBD, potentially enhancing treatment efficacy by merging the anti-inflammatory qualities of the ASC secretome with etanercept's targeted approach to better address the multifaceted pathophysiology of IBD.
Core Tip: This study explores a promising therapeutic strategy for treating inflammatory bowel disease (IBD) by harnessing the potential of a secretome derived from adipose-derived stem cells (ASCs) engineered to produce etanercept, a tumor necrosis factor-blocking drug. The findings demonstrate that the Etanercept-Secretome (Et-Sec) offers enhanced anti-inflammatory effects compared to traditional etanercept treatment. This superior therapeutic potential of the Et-Sec in IBD is attributed to its unique combination of etanercept synthesis and the intrinsic anti-inflammatory and immunomodulatory properties of ASC secretome, making it a promising candidate for advanced IBD therapy.
- Citation: Kim SJ, Kim OH, Hong HE, Ju JH, Lee DS. Etanercept-synthesizing adipose-derived stem cell secretome: A promising therapeutic option for inflammatory bowel disease. World J Gastrointest Surg 2024; 16(3): 882-892
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/882.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.882
Recent advances in cell biology and regenerative medicine have positioned the cell-derived secretome and extracellular vesicles at the forefront of therapeutic interventions, especially concerning inflammatory and infectious diseases. Stem cell-derived secretomes inherently possess anti-inflammatory and immunomodulatory properties, which offer promise in conditions like rheumatoid arthritis[1], autoimmune diseases[2], skin allergies[3,4], and various infectious diseases[5,6]. However, the characteristics of the secretome are not fixed but are instead influenced by the environmental and genetic conditioning of the donor cells[7]. This dynamic nature emphasizes the need for tailored secretomes, optimized for individual disease contexts.
Inflammatory bowel disease (IBD) is a chronic gastrointestinal disorder encompassing Crohn's disease and ulcerative colitis, both marked by persistent inflammation, tissue damage, and immune system dysfunction[8,9]. A pivotal player in the inflammatory cascade of IBD is the pro-inflammatory cytokine, tumor necrosis factor (TNF)-α[10,11]. However, while TNF inhibitors, such as etanercept, have revolutionized the treatment landscape for several autoimmune conditions, their efficacy in IBD remains controversial[12,13]. This is due to the hypothesis that, while TNF-α is a major contributor, it represents only one of several factors involved in the pathogenesis of IBD[8,9,11,12]. This understanding highlights the potential for integrative therapeutic strategies that combine etanercept with other anti-inflammatory agents, offering a more comprehensive therapeutic strategy for IBD. We herein propose constructing etanercept-synthesizing adipse-drived stem cells (ASCs) and using the resulting secretome as a therapeutic agent for the potential therapeutics of IBD. By engineering ASCs to synthesize etanercept, we aim to generate a secretome that includes not only etanercept but also other bioactive molecules with various anti-inflammatory functions, potentially resulting in a synergistic effect on the anti-inflammatory response.
Human ASCs were acquired from Hurim BioCell Co., Seoul, Republic of Korea, institutional review board (IRB) No. 700069-201407-BR-002-01. These ASCs were cultured in DMEM/Low glucose (GibcoBRL, Carlsbad, CA), supplemented with penicillin-streptomycin (GibcoBRL), and incubated at 37 °C in a humidified atmosphere containing 5% CO2. CCD-18co colon normal cells, sourced from the American Type Culture Collection (ATCC; Manassas, VA), were maintained under similar conditions. These cells were cultured in DMEM/high glucose (GibcoBRL) enriched with 10% FBS (Hyclone, Logan, UT) and 1% penicillin/streptomycin (GibcoBRL), also at 37 °C in a humidified 5% CO2 atmosphere.
ZYCY10P3S2T competent cells, transformed with parental plasmids, were incubated overnight at 37 °C in Terrific Broth supplemented with 50 μg/mL kanamycin. A selected single colony was further cultured in Luria broth containing kanamycin for 8 h, followed by a combined incubation in Luria broth with 0.02% arabinose at 30 °C for 5 h. The minicircle DNA was then extracted using the DNA-midi GT plasmid DNA purification kit (Intron Biotechnology, Seongnam, Republic of Korea). For transfection, ASCs were treated with these minicircle vectors employing Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA), adhering to the provided manufacturer’s protocol. Specifically, 5 × 105 ASCs were transfected with mcTNFR2 to facilitate the production of the etanercept-secretome (Et-Sec).
ASCs were cultured in 100 mm cell dishes (Corning Glass Works, Corning, NY). Upon reaching 70%–80% confluence, 1.0 × 106 ASCs were incubated in 7 mL of serum-free low-glucose DMEM for 24 h. To obtain a 0.2 mL volume of secretome from 1.0 × 106 ASCs, the conditioned media were concentrated 25-fold using ultrafiltration units with a 3-kDa molecular weight cutoff (Amicon Ultra-PL 3; Millipore, Bedford, MA).
Total RNA was extracted from CCD-18co cells and mouse liver tissues using TRIzol reagent (Invitrogen, Carlsbad, CA). One µg of RNA was reverse transcribed using a TOYOBO RT-premix kit according to the manufacturer's instructions. For the real-time quantitative polymerase chain reaction (PCR), SYBR Green was used along with the following primers: Mouse TNF-α forward 5′- ACG GCA TGG ATC TCA AAG AC -3′ and reverse 5′- GTG GGT GAG GAG CAC GTA GT -3′; mouse IL-6 forward 5′- AGA AGG AGT GGC TAA GGA CCA A -3′ and reverse 5′- GGC ATA ACG CAC TAG GTT TGC -3′; mouse Monocyte Chemoattractant Protein-1 (MCP-1) forward 5′ -AAC TGC ATC TGC CCT AAG GTC T -3′: Mouse MCP-1 reverse5′ -TGC TTG AGG TGG TTG TGG AA -3′: Mouse GAPDH forward 5′-CGA CTT CAA CAG CAA CTC CCA CTC TTC C-3′ and reverse 5′-TGG GTG GTC CAG GGT TTC TTA CTC CTT-3′: Human TNF-α forward 5′- GGA AGA CCC CTC CCA GAT AG -3′ and reverse 5′- AAC CTC CTC TCT GCC ATC AA-3′; human IL-6 forward 5′- TTT TCT GCC AGT GCC TCT TT -3′ and reverse 5′- CAC ACA GAC AGC CAC TCA CC-3′; human myeloid cell leukemia-1 forward 5’-GGG CAG GAT TGT GAC TCT CAT T-3’ and reverse 5’-GAT GCA GCT TTC TTG GTT TAT GG-3’; Human GAPDH forward 5′- GCA CCG TC AAG GCT GAG AAC - 3′ and reverse 5′- TGG TGA AGA CGC CAG TGG A -3′. Reactions were performed using the Applied Biosystems StepOnePlus real-time PCR system (Thermo, Carlsbad, CA).
CCD-18co normal colon cells and mouse tissues were lysed using the EzRIPA Lysis kit (ATTO Corporation, Tokyo, Japan), with protein concentrations quantified using Bradford reagent (Bio-Rad, Hercules, CA). Western blotting was performed to detect proteins, employing primary antibodies at a 1:1000 dilution from Cell Signaling Technology (Beverly, MA), and HRP-conjugated secondary antibodies at a 1:2000 dilution from Vector Laboratories (Burlingame, CA). The detection of specific immune complexes was facilitated using the Western Blotting Plus Chemiluminescence Reagent (Millipore, Bedford, MA). The primary antibodies targeted MCP-1, TNF-α, interleukin (IL)-1β, IL-6, and β-actin, and the HRP-conjugated secondary antibodies were sourced from Cell Signaling Technology (Beverly, MA).
Formalin-fixed and paraffin-embedded tissue sections underwent deparaffinization and rehydration through an ethanol series. This was followed by epitope retrieval using established protocols. For immunohistochemical staining, antibodies targeting TNF-α, PECAM-1, F4/80, and MCP-1 were utilized, all of which were procured from Cell Signaling Technology. The stained samples were then examined under a laser-scanning microscope (Eclipse TE300; Nikon, Tokyo, Japan) to assess antibody expression.
Five-week-old male BALB/c mice, sourced from Orient Bio (Seongnam, Republic of Korea), were used in this animal experiment, conducted in compliance with the Institute for Laboratory Animal Research guidelines at the Catholic University of Korea (IRB No. CUMC- 2022-0020-01). To establish an acute experimental IBD model, BALB/c mice were given 1% dextran sulfate sodium (DSS) or saline in their drinking water for three weeks. Control mice (n = 8) and 1% DSS-treated mice (n = 30) were administered injections of 0.1 mL normal saline (NS) (n = 10), 0.1 mL control secretome (Ct-Sec, n = 10), and 0.1 mL Et-Sec (n = 10), respectively. In the secretome groups, a 0.1 mL volume of the secretome, equivalent to the amount obtained from 5 × 105 ASCs, was intravenously administered. These injections were given twice weekly for two weeks. Following the treatment period, the mice were euthanized, and their colons were immediately harvested for length measurement, Western blot analysis, and histological examination.
Blood samples collected from each mouse were centrifuged for 15 min at 750 g to obtain serum. The levels of mouse IL-6, MCP-1, and TNF-α in the serum were then quantified using ELISA kits (Biolegend, San Diego, CA), following the manufacturer's instructions.
Data analysis was performed using SPSS 11.0 software (SPSS Inc., Chicago, IL, United States), with results presented as mean ± SD. The Kruskal–Wallis test was utilized for statistical comparisons among groups, and a probability value of P < 0.05 were considered statistically significant.
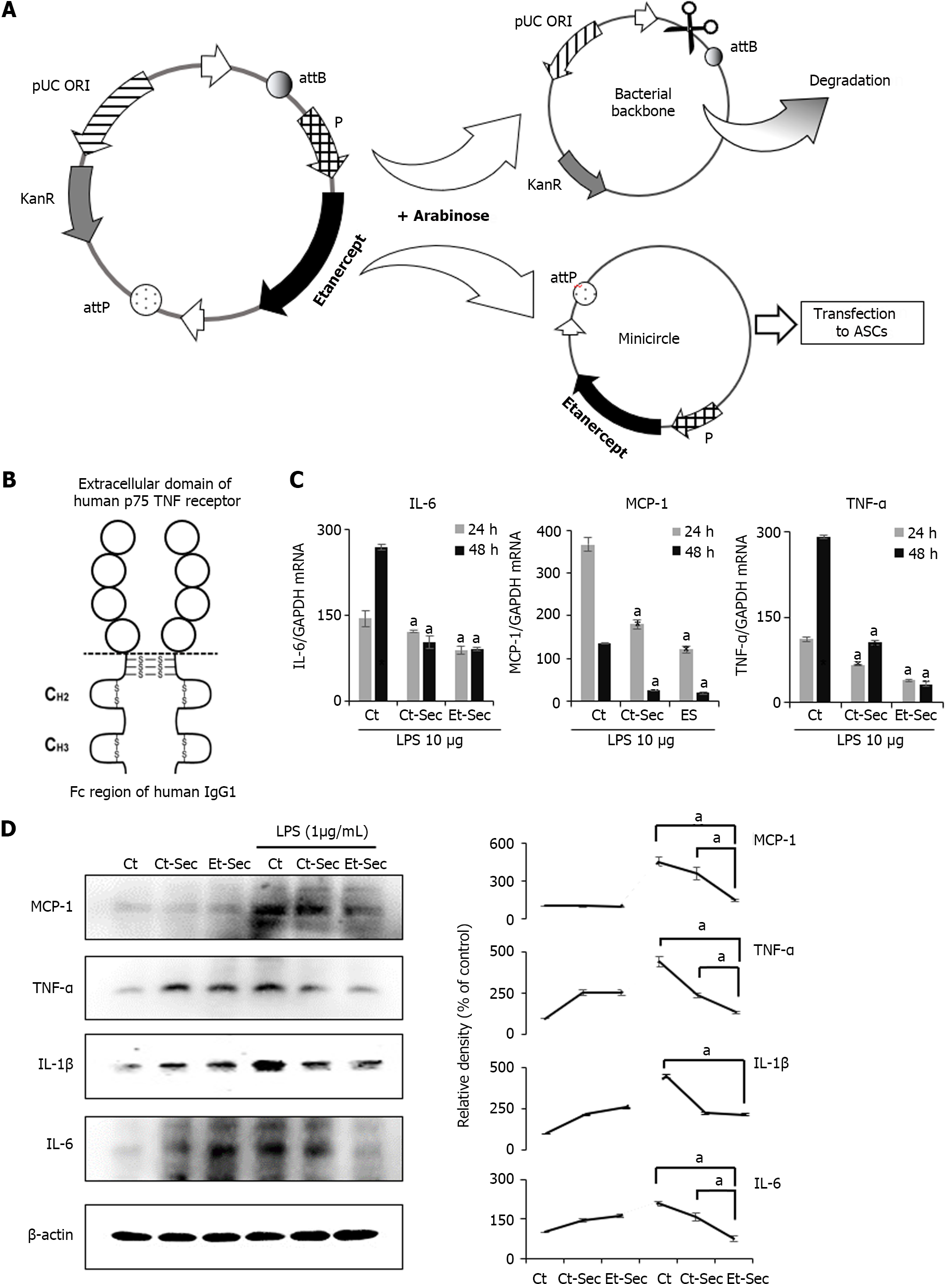
The mini-circle plasmid encoding etanercept was derived from the parental plasmid through treatment with arabinose (Figure 1A). Secretome samples were collected from an ASC cell line subjected to either etanercept gene insert-containing minicircle transfection or left untransfected. The secretome obtained from the untransfected cell line was designated as Ct-Sec, while that from the etanercept-transfected cell line was termed Et-Sec. Both samples were concentrated 25-fold. Etanercept is a protein-based drug with two domains, comprising a TNF-α receptor domain and an immunoglobulin G1 Fc domain, enabling it to bind and neutralize TNF-α, a critical inflammatory cytokine (Figure 1B).
To assess the impact of these secretome samples on inflammatory markers, the CCD-18Co colon normal cell line was treated with 1 ng/mL of lipopolysaccharide (LPS) and either Ct-Sec or Et-Sec. After 24 and 48 h, cells were harvested, and mRNA was extracted to quantify the expression levels of inflammatory markers TNF-α and IL-6. The findings indicated that, irrespective of LPS treatment, the mRNA expression levels of TNF-α and IL-6 were significantly lower in the Et-Sec group compared to the other groups, including the control (no treatment), Ct-Sec, and Et-Sec groups (Ps < 0.05; Figure 1C).
Subsequently, Western blot analysis of inflammatory markers MCP-1, TNF-α, IL-1β, and IL-6 was conducted on the CCD-18Co colon normal cell line, both in the absence and presence of 1 ng/mL LPS treatment, while exposing the cells to either Ct-Sec or Et-Sec samples (Figure 1D). After treatment with En-Sec, there was a significant reduction (Ps < 0.05) in the expression levels of the inflammatory cytokines MCP-1, TNF-α, and IL-6, when compared to the Ct-Sec group. In addition, the expression levels of IL-1β in both the Ct-Sec and En-Sec groups remained relatively similar. Overall, these findings suggest that the Et-Sec group possesses a more substantial anti-inflammatory effect in the context of LPS-induced inflammation in the CCD-18Co colon normal cell line, as compared to the Ct-Sec group.
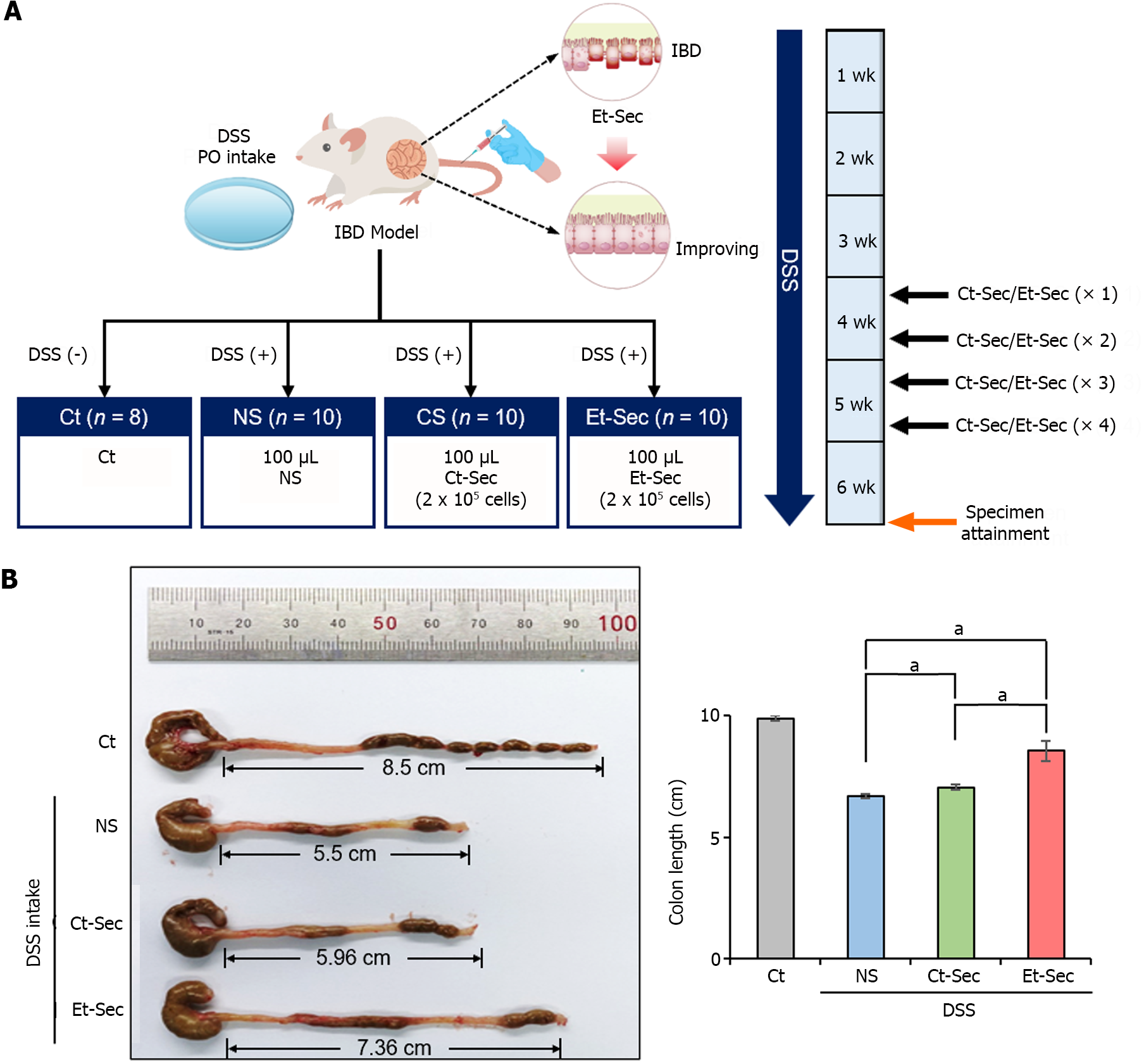
DSS-induced IBD mouse model was generated by administering DSS (2.5 g/250 mL) in the drinking water, resulting in intestinal epithelial damage and inflammation. Subsequently, 100 μL of NS, Ct-Sec (1 × 106 cells) or Et-Sec (1 × 106 cells) were administered via the tail vein four times a week (total 400 μL) into the DSS-induced IBD mouse (n = 10 per group), respectively (Figure 2A). In the DSS-induced IBD mouse model, a shortened colon or small intestine length indicates inflammation and tissue damage, while an increase in bowel length suggests a healthier gut. After six weeks, the mice were euthanized, and their bowel length was measured to evaluate the severity of inflammation and tissue damage. In the DSS intake groups (NS, Ct-Sec, and Et-Sec), bowel length shortening was observed compared to the control group (Ct) (Figure 2B). Among the DSS intake groups, the Secretome-treated groups (Ct-Sec and Et-Sec) exhibited a significantly more pronounced bowel length elongation compared to the NS group (Ps < 0.05). When comparing the Ct-Sec group with the Et-Sec group, Et-Sec showed a significantly greater bowel length elongation (P < 0.05). These results suggest that Et-Sec demonstrates an enhanced anti-inflammatory effect in an in vivo mouse model of IBD compared to Ct-Sec.
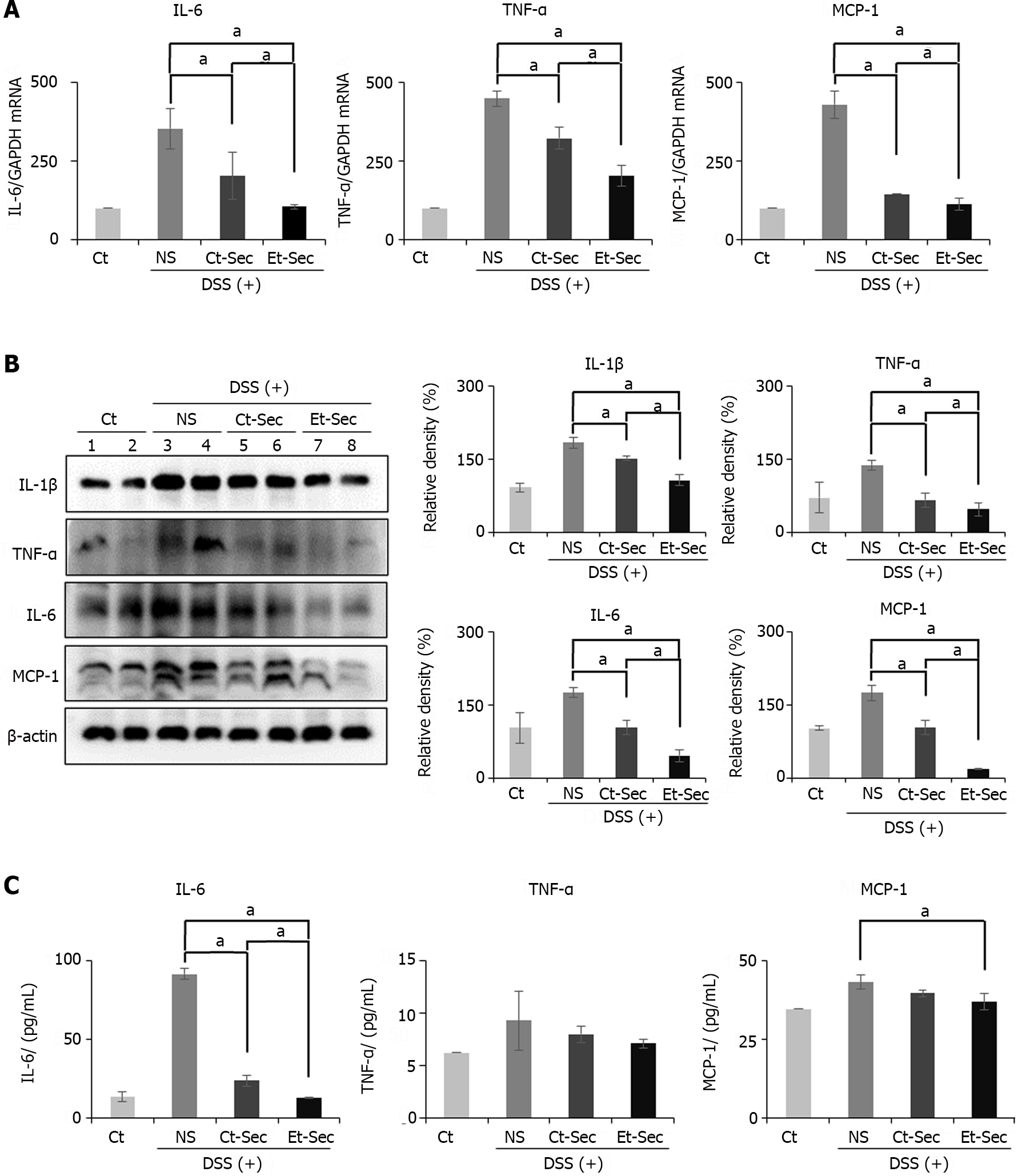
Following euthanasia, intestinal tissue was collected from the mice, and Real-time PCR was performed to assess the mRNA expression of inflammatory mediators, including IL-6, TNF-α, and MCP-1 (Figure 3A). In the DSS intake groups (NS, Ct-Sec, and Et-Sec), the mRNA expression of these inflammatory mediators significantly increased (Ps < 0.05). Among the DSS intake groups, the Secretome-treated groups (Ct-Sec and Et-Sec) showed a significant reduction in mRNA expression compared to the NS group (Ps < 0.05). Within the Secretome-treated groups, Et-Sec exhibited a more pronounced decrease in mRNA expression of IL-6, TNF-α, and MCP-1 compared to Ct-Sec (P < 0.05).
Subsequently, Western blot analysis was conducted to compare the expression of inflammatory proteins, including IL-1β, TNF-α, IL-6, and MCP-6 in the intestinal tissues of each group (Figure 3B). DSS intake resulted in a significant increase in the expression of the proteins compared to the Ct (Ps < 0.05). Among the DSS intake groups, the Secretome-treated groups (Ct-Sec and Et-Sec) exhibited a significant reduction of the inflammatory markers compared to the NS group (Ps < 0.05). Within the Secretome-treated groups, Et-Sec showed a more substantial reduction in the expression of IL-1β, TNF-α, IL-6, and MCP-6 compared to Ct-Sec (Ps < 0.05).
Next, blood samples were collected from each group and the levels of serum inflammatory markers, including IL-6, TNF-α, and MCP-1, were measured using an ELISA (Figure 3C). In the case of IL-6 serum levels, there was a substantial increase following DSS intake, which was significantly reduced in the secretome-treated groups (Ct-Sec and Et-Sec; P < 0.05), with Et-Sec demonstrating a more pronounced reduction (P < 0.05). For TNF-α serum levels, the secretome-treated groups showed a decrease, although it was not statistically significant. In the case of MCP-1 serum levels, among the secretome-treated groups, the Et-Sec group exhibited a significant reduction in serum concentration compared to the NS group (P < 0.05).
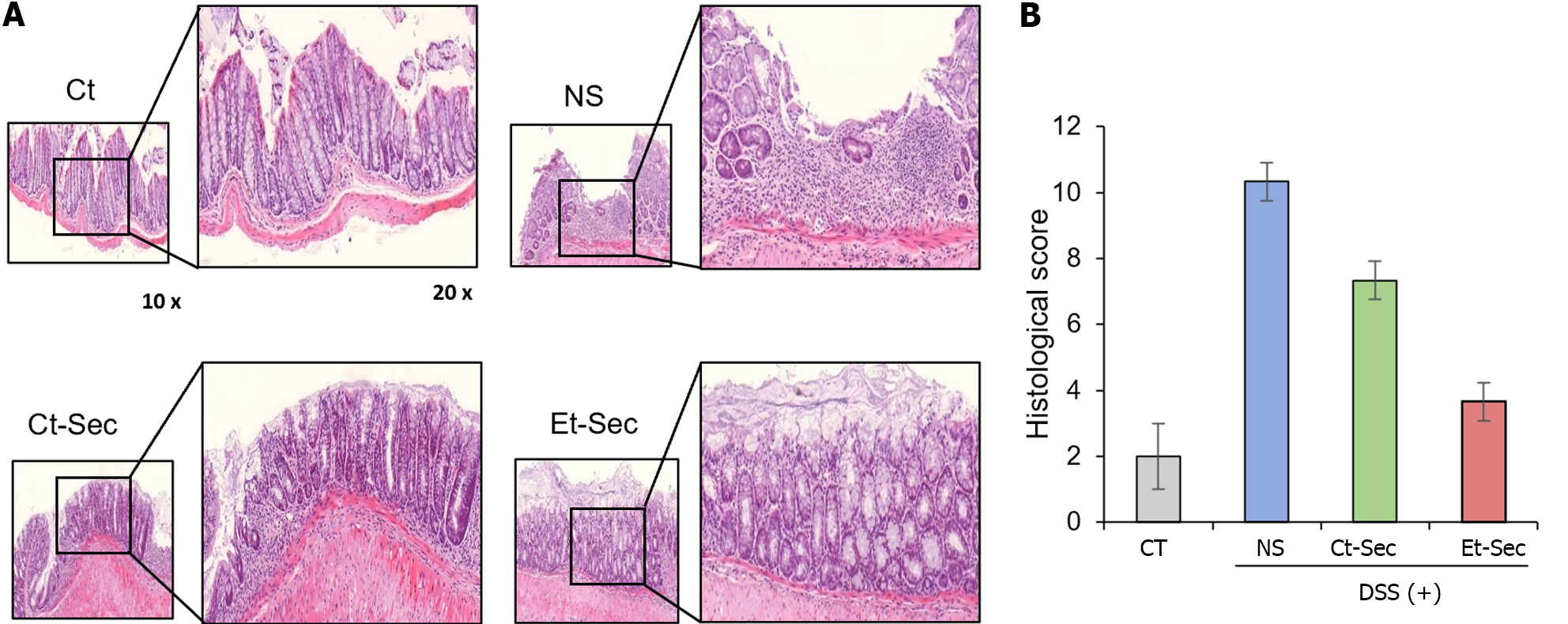
Histological and immunohistochemical analyses were performed on intestinal tissue samples obtained from each group. Hematoxylin and eosin (H&E) staining revealed that the intestinal tissue, which exhibited disorganization following DSS administration, showed a marked recovery of tissue architecture in the Et-Sec group, indicating a potential therapeutic effect of the Et-Sec on IBD (Figure 4A left). In the comparison of histological scores reflecting inflammation in H&E stains, it was observed that the Et-Sec group exhibited significantly lower scores compared to all the DSS-treated groups (P < 0.05; Figure 4A right).
Additionally, investigations and comparisons of inflammatory markers, namely TNF-α, PECAM-1, F4/80, and MCP-1, were conducted within each group using immunohistochemical stains (Figure 4B). DSS intake significantly increased these inflammatory markers, while secretome treatments (Ct-Sec and Et-Sec) significantly reduced the elevated inflammatory markers (P < 0.05). Furthermore, in all cases, Et-Sec treatment led to a more significant reduction in inflammatory markers compared to Ct-Sec treatment (P < 0.05). Taken together, these findings provide evidence that the Et-Sec exerts a beneficial effect on IBD inflammation, highlighting its potential therapeutic utility in the treatment of IBD.
This study focused on the potential anti-inflammatory effects of the secretome obtained from ASCs synthesizing etanercept on IBD using both CCD-18Co colon normal cell line with LPS-induced toxicity and DSS-induced IBD mouse model. In the in vitro using CCD-18Co colon normal cells with LPS-induced toxicity, it showed significant reductions in TNF-α and IL-6 mRNA expression levels, along with decreased inflammatory cytokines MCP-1, TNF-α, IL-1β, and IL-6 through Western blot analysis. These findings indicated potent anti-inflammatory properties. In the DSS-induced IBD mouse model, intraveous administration of Et-Sec led to noticeable bowel length elongation, signifying reduced inflammation and tissue damage. Real-time PCR and Western blot analysis on intestinal tissues further supported these results, showing decreased mRNA expression and protein levels of inflammatory mediators.
Serum inflammatory markers were assessed via ELISA, revealing that the Et-Sec group had the lowest levels and histological evaluations confirmed the markedly reduced inflammation in the Et-Sec group. In summary, this study provides robust evidence for the potent anti-inflammatory effects of Et-Sec, both in vitro and in vivo, suggesting its promising candidacy for treating IBD.
TNF-α plays substantial role in the pathogenesis of IBD, such as Crohn's disease and ulcerative colitis[10,11]. In IBD, the intestinal mucosa is exposed to a variety of stimuli, such as luminal antigens and commensal bacteria, that trigger an aberrant immune response[8,9]. This results in the recruitment of immune cells, including macrophages, dendritic cells, and T cells, to the gut tissue, and the subsequent release of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IL-12[8,9,11,14]. Among these cytokines, TNF-α serves as a central regulator, triggering the production of pro-inflammatory cytokines, chemokines, and adhesion molecules, as well as facilitating the recruitment and activation of immune cells, including neutrophils and monocytes[10,11]. This further perpetuates the inflammatory response, resulting in tissue damage, ulceration, and fibrosis, which can ultimately lead to intestinal strictures and bowel obstruction. In addition to its direct effects on immune cells, TNF-α also plays a role in disrupting the intestinal barrier function, which can result in increased permeability and translocation of bacteria and antigens across the epithelial barrier[8,9,11]. This further amplifies the inflammatory response and can contribute to the development of systemic inflammation and extra-intestinal manifestations of IBD[8,9,11].
Etanercept, a potent TNF-α inhibitor, has been shown to be effective in treating certain inflammatory conditions, such as rheumatoid arthritis and psoriasis[15,16]. However, its effectiveness in treating IBD has been variable and less consistent[12,13]. One possible explanation for this is that TNF-α is just one of many pro-inflammatory cytokines that contribute to the pathogenesis of IBD, and targeting TNF-α alone may not be sufficient to fully suppress the inflammatory response[8,9,11,12]. In addition, IBD is a complex and heterogeneous disease with diverse underlying mechanisms and multiple cell types involved in the inflammatory process, which may require a more multi-targeted and individualized approach for effective treatment[8,9]. Another potential reason is that etanercept may have limited efficacy in the gut due to its relatively large molecular size, which may limit its penetration into the intestinal tissue[17,18]. In contrast, smaller molecules or biological drugs that are more gut-selective, such as vedolizumab or ustekinumab, may have a greater therapeutic effect in IBD[19,20]. Overall, while etanercept may provide some benefit in the treatment of IBD, its effectiveness may be limited due to the complex and multifactorial nature of the disease, and the potential challenges in achieving adequate drug distribution to the gut tissue.
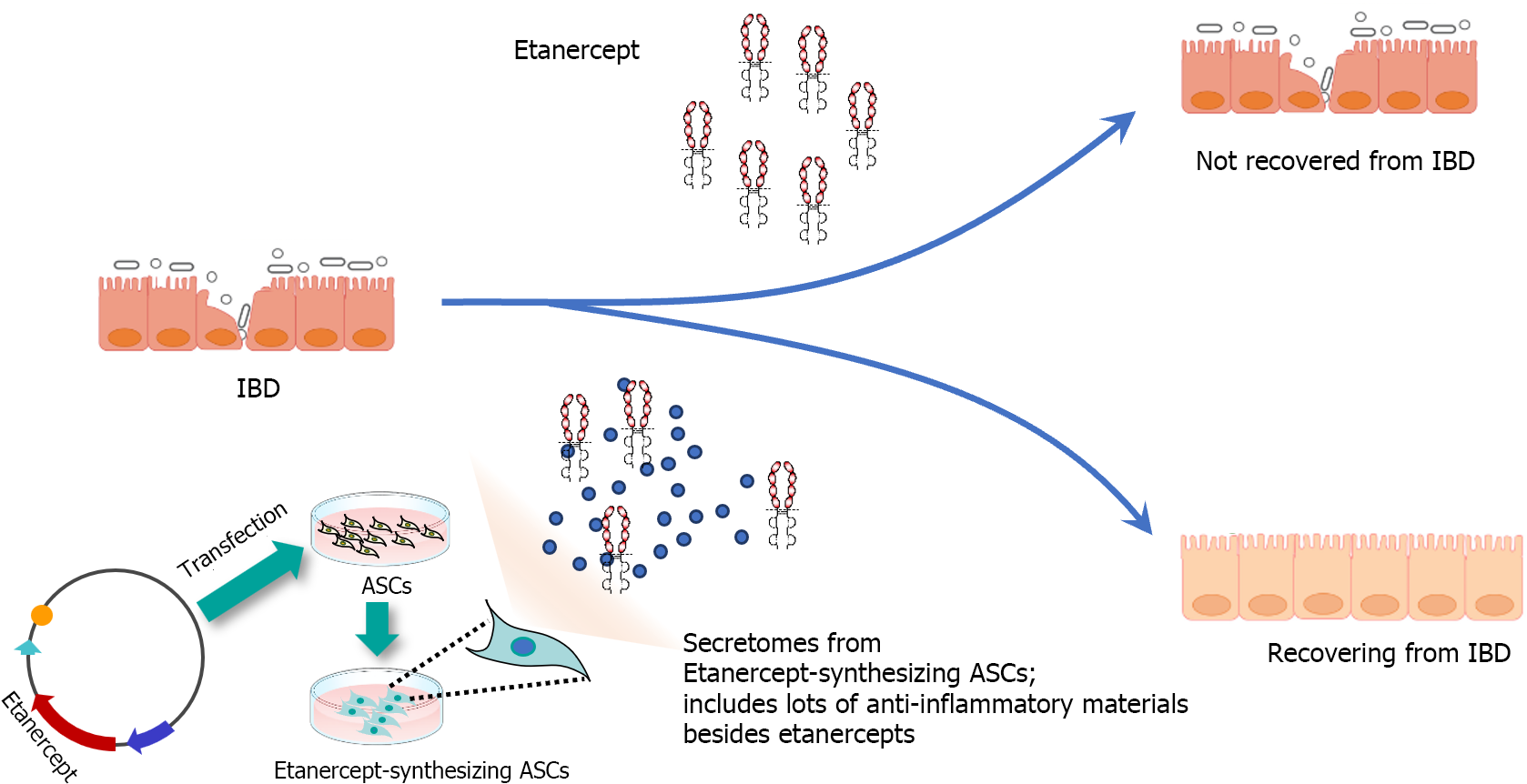
In response to the valuable insights provided by the reviewer, we have incorporated the following paragraph into the revised manuscript; The therapeutic efficacy of Etanercept in IBD has been a subject of debate, with evidence suggesting its potential to induce IBD in the treatment of other autoimmune conditions such as psoriasis[21,22]. However, it is important to differentiate between the effects of Etanercept alone and ASC-derived Et-Sec. ASCs are renowned for their anti-inflammatory and immunomodulatory properties, further enhanced by their secretion of a wide array of bioactive molecules, including cytokines, growth factors, and extracellular vesicles. These molecules play a crucial role in regulating immune responses and facilitating tissue regeneration. Et-Sec represents the secretome of ASCs, specifically engineered to produce etanercept, with its principal component being the augmented ASC secretome, not etanercep, hypothesizing a synergistic effect that could more effectively tackle the intricate pathophysiology of IBD than Etanercept alone (Figure 5). While etanercept targets TNFα directly, the ASC secretome can modulate multiple inflammatory pathways and cellular interactions. This approach not only targets TNF-α directly but also modulates multiple inflammatory pathways and cellular interactions, offering a comprehensive modulation of the inflammatory environment characteristic of IBD. Furthermore, the role of the ASC secretome in tissue repair and regeneration may provide additional therapeutic benefits in IBD treatment, particularly in managing chronic inflammation-induced tissue damage and promoting the healing and restoration of intestinal mucosa integrity.
This study extensively investigated the anti-inflammatory potential of Et-Sec, secretome obtained from ASCs synthesizing etanercept, in the context of IBD. In both in vitro and in vivo models of IBD, notable reductions in multiple inflammatory markers, such as TNF-α, MCP-1, IL-1β, and IL-6, were consistently observed within the Et-Sec group as compared to the Ct-Sec group. Additionally, intravenous Et-Sec administration in the IBD mouse model led to noticeable bowel length elongation, indicating reduced inflammation and tissue damage. These findings collectively underscore the robust anti-inflammatory effects of Et-Sec, positioning it as a promising candidate for IBD treatment.
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the gastrointestinal tract, significantly influenced by tumor necrosis factor (TNF)-α. Etanercept, a TNF decoy receptor, along with the anti-inflammatory secretome from adipose-derived stem cells (ASCs), offers a novel approach for IBD therapy.
This study aims to address the limitations of current IBD treatments by exploring the combined anti-inflammatory effects of ASC-derived secretome and etanercept, potentially offering a more comprehensive and effective treatment strategy for IBD.
To investigate the anti-inflammatory efficacy of the secretome from etanercept-synthesizing ASCs in colon cells and a dextran sulfate sodium (DSS)-induced IBD mouse model, assessing its potential as a novel therapeutic agent for IBD treatment.
ASCs were transfected with etanercept-encoding plasmids, producing a specialized secretome. This Etanercept-secretome (Et-Sec) was evaluated for anti-inflammatory effects both in vitro in colon cells and in vivo in a DSS-induced IBD mouse model.
Et-Sec group, treated with the secretome from etanercept-synthesizing ASCs, showed a substantial reduction in the expression of inflammatory mediators, including interleukin-6, Monocyte Chemoattractant Protein-1, and TNF-α, relative to the control secretome group. Furthermore, the Et-Sec group displayed a significant therapeutic benefit by better preserving the structure of intestinal tissues, highlighting its potential in treating inflammatory bowel disease.
The study concludes that the Et-Sec from ASCs significantly reduces inflammatory markers and mitigates tissue damage in IBD, demonstrating its potential as an effective therapeutic agent for IBD treatment.
Future research should focus on further validating the efficacy of Et-Sec in diverse IBD models and exploring its potential as a comprehensive treatment strategy for various forms of IBD.
We express our gratitude to Jeong-Yeon Seo for manuscript processing and to Jennifer Lee for the contributions in illustration work.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rathnaswami A, India S-Editor: Li L L-Editor: A P-Editor: Cai YX
| 1. | Colombini A, Libonati F, Lopa S, Ragni E, De Luca P, Zagra L, Sinigaglia F, Moretti M, de Girolamo L. Immunomodulatory potential of secretome from cartilage cells and mesenchymal stromal cells in an arthritic context: From predictive fiction toward reality. Front Med (Lausanne). 2022;9:992386. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 2. | Műzes G, Sipos F. Mesenchymal Stem Cell-Derived Secretome: A Potential Therapeutic Option for Autoimmune and Immune-Mediated Inflammatory Diseases. Cells. 2022;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 49] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 3. | Yang J, Xiao M, Ma K, Li H, Ran M, Yang S, Yang Y, Fu X. Therapeutic effects of mesenchymal stem cells and their derivatives in common skin inflammatory diseases: Atopic dermatitis and psoriasis. Front Immunol. 2023;14:1092668. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 4. | Shin TH, Kim HS, Choi SW, Kang KS. Mesenchymal Stem Cell Therapy for Inflammatory Skin Diseases: Clinical Potential and Mode of Action. Int J Mol Sci. 2017;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Ardalan M, Chodari L, Zununi Vahed S, Hosseiniyan Khatibi SM, Eftekhari A, Davaran S, Cucchiarini M, Roshangar L, Ahmadian E. Stem cell-derived biofactors fight against coronavirus infection. World J Stem Cells. 2021;13:1813-1825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Razi S, Molavi Z, Mirmotalebisohi SA, Niknam Z, Sameni M, Niazi V, Adibi A, Yazdani M, Ranjbar MM, Zali H. Mesenchymal Stem Cells in the Treatment of New Coronavirus Pandemic: A Novel Promising Therapeutic Approach. Adv Pharm Bull. 2022;12:206-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | L PK, Kandoi S, Misra R, S V, K R, Verma RS. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 227] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 8. | Wehkamp J, Götz M, Herrlinger K, Steurer W, Stange EF. Inflammatory Bowel Disease. Dtsch Arztebl Int. 2016;113:72-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Tarris G, de Rougemont A, Charkaoui M, Michiels C, Martin L, Belliot G. Enteric Viruses and Inflammatory Bowel Disease. Viruses. 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 524] [Cited by in F6Publishing: 525] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 11. | Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR, Yang SH. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int J Mol Sci. 2021;22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 424] [Article Influence: 141.3] [Reference Citation Analysis (0)] |
| 12. | Pugliese D, Felice C, Papa A, Gasbarrini A, Rapaccini GL, Guidi L, Armuzzi A. Anti TNF-α therapy for ulcerative colitis: current status and prospects for the future. Expert Rev Clin Immunol. 2017;13:223-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 70] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 13. | Mazhar F, Battini V, Pozzi M, Invernizzi E, Mosini G, Gringeri M, Capuano A, Scavone C, Radice S, Clementi E, Carnovale C. Changes in Anthropometric Parameters After Anti-TNFα Therapy in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. BioDrugs. 2020;34:649-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Saez A, Herrero-Fernandez B, Gomez-Bris R, Sánchez-Martinez H, Gonzalez-Granado JM. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int J Mol Sci. 2023;24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 58] [Reference Citation Analysis (0)] |
| 15. | Haraoui B, Bykerk V. Etanercept in the treatment of rheumatoid arthritis. Ther Clin Risk Manag. 2007;3:99-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Culy CR, Keating GM. Etanercept: an updated review of its use in rheumatoid arthritis, psoriatic arthritis and juvenile rheumatoid arthritis. Drugs. 2002;62:2493-2537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Sandborn WJ, Hanauer SB, Katz S, Safdi M, Wolf DG, Baerg RD, Tremaine WJ, Johnson T, Diehl NN, Zinsmeister AR. Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001;121:1088-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 663] [Cited by in F6Publishing: 614] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 18. | Oh J, Arkfeld DG, Horwitz DA. Development of Crohn's disease in a patient taking etanercept. J Rheumatol. 2005;32:752-753. [PubMed] [Cited in This Article: ] |
| 19. | Shim HH, Chan PW, Chuah SW, Schwender BJ, Kong SC, Ling KL. A review of vedolizumab and ustekinumab for the treatment of inflammatory bowel diseases. JGH Open. 2018;2:223-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Honap S, Meade S, Ibraheim H, Irving PM, Jones MP, Samaan MA. Effectiveness and Safety of Ustekinumab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2022;67:1018-1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | Korzenik J, Larsen MD, Nielsen J, Kjeldsen J, Nørgård BM. Increased risk of developing Crohn's disease or ulcerative colitis in 17 018 patients while under treatment with anti-TNFα agents, particularly etanercept, for autoimmune diseases other than inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50:289-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Tolu S, Rezvani A, Hindioglu N, Calkin Korkmaz M. Etanercept-induced Crohn's disease in ankylosing spondylitis: a case report and review of the literature. Rheumatol Int. 2018;38:2157-2162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |