Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.871
Peer-review started: December 5, 2023
First decision: January 4, 2024
Revised: January 12, 2024
Accepted: February 28, 2024
Article in press: February 28, 2024
Published online: March 27, 2024
Currently, the primary treatment for gastroesophageal reflux is acid suppression with proton pump inhibitors, but they are not a cure, and some patients don’t respond well or refuse long-term use. Therefore, alternative therapies are needed to understand the disease and develop better treatments. Laparoscopic anti-reflux surgery (LARS) can resolve symptoms of these patients and plays a significant role in evaluating esophageal healing after preventing harmful effects. Successful LARS improves typical gastroesophageal reflux symptoms in most patients, main
To explore the role of inflammatory biomolecules in LARS and assess the time required for esophageal epithelial recovery.
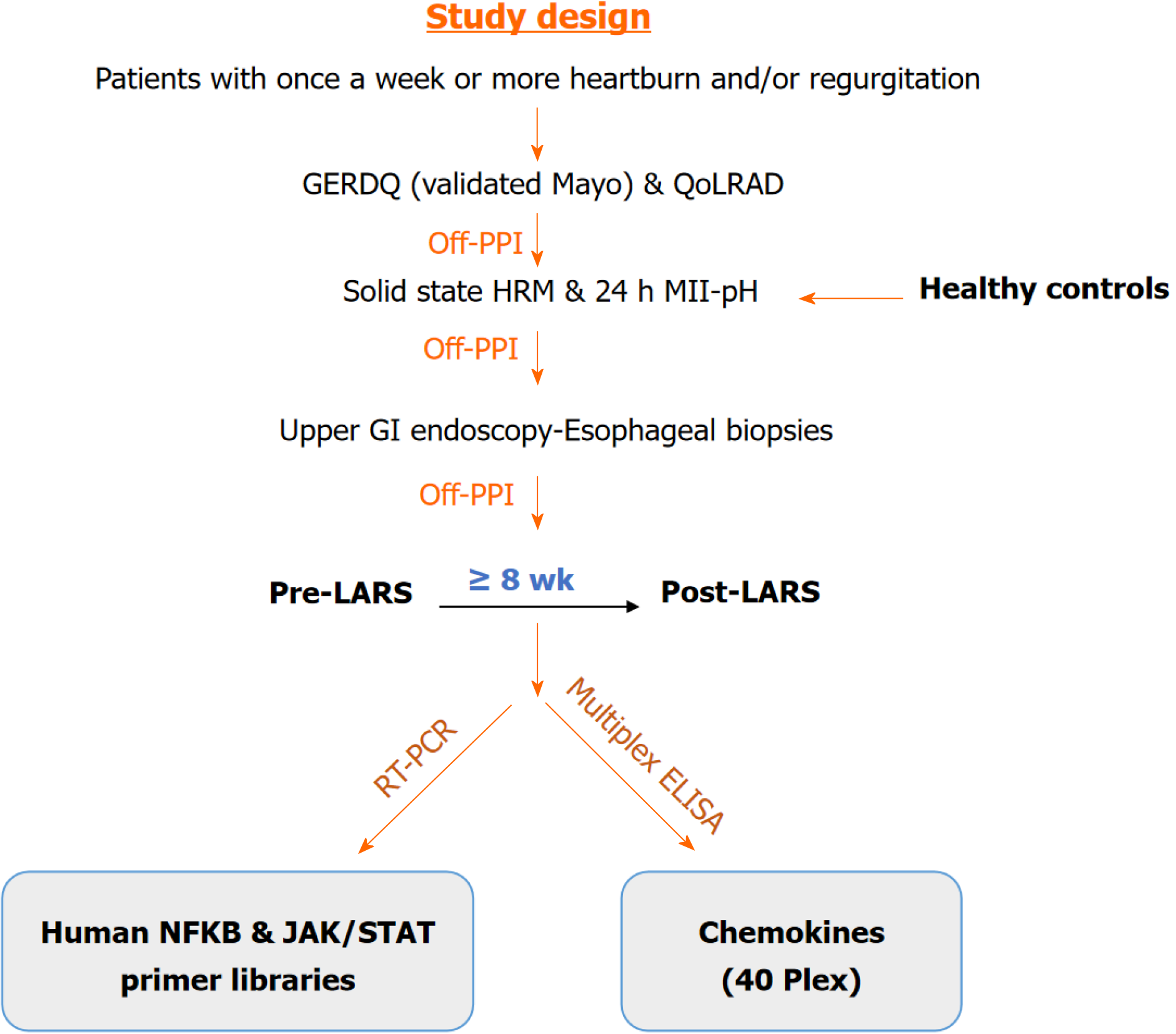
Of 22 patients with LARS (pre- and post/5.8 ± 3.8 months after LARS) and 25 healthy controls (HCs) were included. All subjects underwent 24-h multichannel intraluminal impedance-pH monitoring and upper gastrointestinal endoscopy, during which esophageal biopsy samples were collected using endoscopic tech
Post-LARS samples showed significant increases in proinflammatory cytokines [interleukin (IL)-1β, interferon-γ, C-X-C chemokine ligand 2 (CXCL2)], anti-inflammatory cytokines [CC chemokine ligand (CCL) 11, CCL13, CCL17, CCL26, CCL1, CCL7, CCL8, CCL24, IL-4, IL-10], and homeostatic cytokines (CCL27, CCL20, CCL19, CCL23, C
The presence of proinflammatory proteins post-LARS suggests ongoing inflammation in the epithelium. Elevated homeostatic cytokine levels indicate cell balance is maintained for about 6 months after LARS. The anti-inflammatory response post-LARS shows suppression of inflammatory damage and ongoing postoperative recovery.
Core Tip: Even six months after laparoscopic anti-reflux surgery, specific pro-inflammatory cytokines continue to exhibit activity. Elevated levels of anti-inflammatory and regulatory cytokines suggest their involvement in preserving cellular homeostasis and regulating inflammation. As a precaution, we recommend that patients who have undergone laparoscopic anti-reflux surgery avoid refluxogenic foods to prevent short-term gastroesophageal reflux disease symptoms.
- Citation: Ergun P, Kipcak S, Selvi Gunel N, Yildirim Sozmen E, Bor S. Inflammatory responses in esophageal mucosa before and after laparoscopic antireflux surgery. World J Gastrointest Surg 2024; 16(3): 871-881
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/871.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.871
Gastroesophageal reflux disease (GERD) is a chronic public health problem characterized by typical symptoms of heart
The primary treatment modality currently is inhibiting gastric acid secretion with proton pump inhibitors (PPIs). How
Since symptom resolution can be achieved in up to 93.1% of patients following laparoscopic anti-reflux surgery (LARS)[6], this modality is crucial for assessing the healing process of the esophageal epithelium after preventing the effects of noxious agents. The aim of this study was to investigate the role of inflammatory and recovery biomolecules after LARS by exploring the inflammatory pathways that may contribute to the pathogenesis of the disease. Additionally, we aimed to determine healing time frame to ascertain whether a meaningful period for healing allows the esophageal epithelium to fully recover.
In total, 35 patients with GERD who had been approved for LARS by the Ege University GERD Study Group, and 25 healthy controls (HCs) were included in the study. However, the follow-up upper gastrointestinal (GI) endoscopy con
Esophageal motility tests were done before placing the multichannel intraluminal impedance-pH (MII-pH) catheter at the upper lower esophageal sphincter (LES) boundary. Data were analyzed using MMS software version 8.1 (MMS - Laborie, the Netherlands). An eight-channel motility catheter with four radial and four circumferential openings was used for motility measurements. After an 8-h fast, the catheter was placed 50-55 cm deep via the nasal passage. LES loca
The exclusion criteria for both patients and HCs included primary esophageal motility disorders, Barrett’s esophagus, previous upper GI surgery, chronic renal failure, severe coronary artery disease, severe chronic obstructive pulmonary disease, uncontrolled diabetes mellitus, pregnancy, lactation, and other disorders that may affect the study, with the ex
Upper GI endoscopy was conducted by one gastroenterologist (Bor S), and the biopsy samples were taken by one tech
The biopsy samples were homogenized using a Bioprep-6 Homogenizer (Hangzhou Allsheng Instruments Inc., Zhejiang, China), and total RNA was isolated with an Aurum™ Total RNA Mini Kit (Bio-Rad Laboratories, Inc., Hercules, CA) following to the manufacturer’s instructions. The absorbance, indicating the concentration and purity of the total RNA, was measured at 260/280 nm with a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE) using 2 μL of each homogenized and isolated sample.
cDNA was synthesized from total RNA in each sample using qPCR and an iScript cDNA Synthesis Kit with a reverse transcriptase enzyme (Bio-Rad Laboratories, Inc., Hercules, CA) following to the manufacturer’s instructions. Real-time polymerase chain reaction was conducted using a LightCycler® 480 (Roche Diagnostics Inc., Basel, CH). iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA) and two different primer libraries - (Human JAK/STAT Signaling Primer Library and Human NFκB Primer Library) Real Time Primers (LLC) - were employed according to the manufacturer’s specifications. The housekeeping genes selected were actin-beta, beta-2-microglobulin, and ribosomal protein L13a.
The biopsy samples were homogenized using a Bioprep-6 Homogenizer (Hangzhou Allsheng Instruments Inc., Zhejiang, China), and total protein was extracted with a Bio-Plex TM Cell Lysis Kit (Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer’s instructions. After centrifugation (4500 rpm for 10 min)[7], the isolated proteins were divided into aliquots, and protein amounts were determined using the Lowry method[7]. The protein levels of che
The 2-ΔΔCt method was used for the quantitation analysis of gene expression. The corresponding gene expression levels in each group were compared. Gene expression levels in each group were compared, and genes with a fold change ≥ 1.5 were included in the evaluation. Statistical analyses were performed using ANOVA, Student’s t test (for parametric data) and the Mann-Whitney U test (for nonparametric data) with IBM® SPSS® Statistics 25.0. A P value of < 0.05 was con
One patient with erosive reflux disease (ERD) C/D out of 23 patients and 5 out of 25 HCs were excluded for various reasons: The presence of multiple polyps observed during upper GI endoscopy, excessive bleeding during biopsies, desaturation, and other related issues with the sedation procedure, as well as the relapse of erosions and/or symptoms after LARS. Ultimately, a total of 22 patients [10 ERD A/B, 6 ERD C/D, 6 non-ERD (NERD)] and 20 HCs were included in the study (Table 1).
| Group | n | Gender | Age | BMI |
| GERD | 22 | 9 females/13 males | 42.9 ± 11.5 | 25.1 ± 2.5 |
| HC | 20 | 13 females/7 males | 41.9 ± 10.8 | 24.5 ± 1.8 |
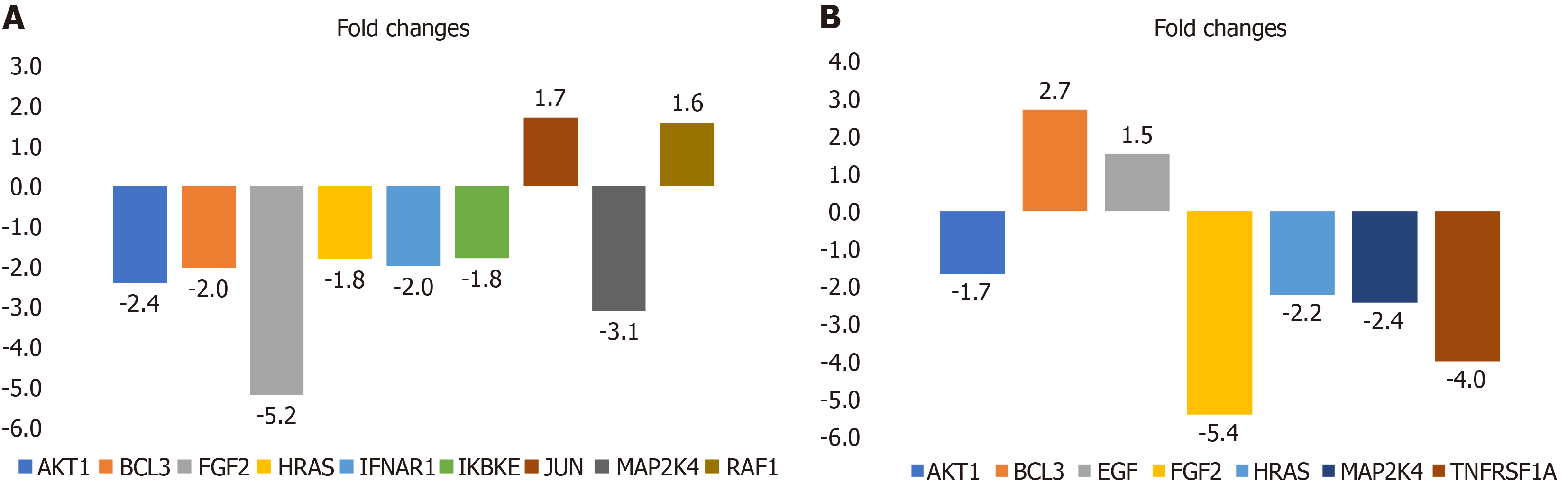
CC chemokine ligand (CCL)2 (-2.3-fold) and epidermal growth factor (EGF) (-2.2-fold) gene expression levels were lower in the pre-LARS group compared to the HC group (Supplementary Table 1). On the other hand, mRNA expression levels of JUN (1.7-fold) and RAF1 (1.6-fold) were increased, while those of fibroblast growth factor 2 (FGF2) (-5.2-fold), mitogen-activated protein kinase 4 (MAP2K4) (-3.1-fold), EP300 (-2.8-fold), MCM5 (-2.8-fold), AKT1 (-2.4-fold), IRF9 (-2.3-fold), PIK3R2 (-2.3-fold), MYC (-2.2-fold), B-cell CLL/lymphoma 3 (BCL3) (-2.0-fold), PIAS4 (-2.0-fold), interferon (IFN) (alpha, beta and omega) receptor 1 (-2.0-fold), HRAS (-1.8-fold), IKBKE (-1.8-fold), RELA (-1.8-fold), TICAM1 (-1.5-fold), and PTPN11 (-1.5-fold) were decreased in the post-LARS group compared to levels in HCs (Figure 2A and Supplementary Table 2).
The fold changes in the post-LARS group compared to the pre-LARS group depicted in Figure 2B and Supplementary Table 3. While EGF (1.5-fold) and BCL3 (2.7-fold) expression increased after LARS, FGF2 (-5.4-fold), RIPK1 (-4.6-fold), tumor necrosis factor receptor superfamily, member 1 (-4.0-fold), MAP2K4 (-2.4-fold), HRAS (-2.2-fold) and AKT1 (-1.7 fold) decreased compared to pre-LARS measurements.
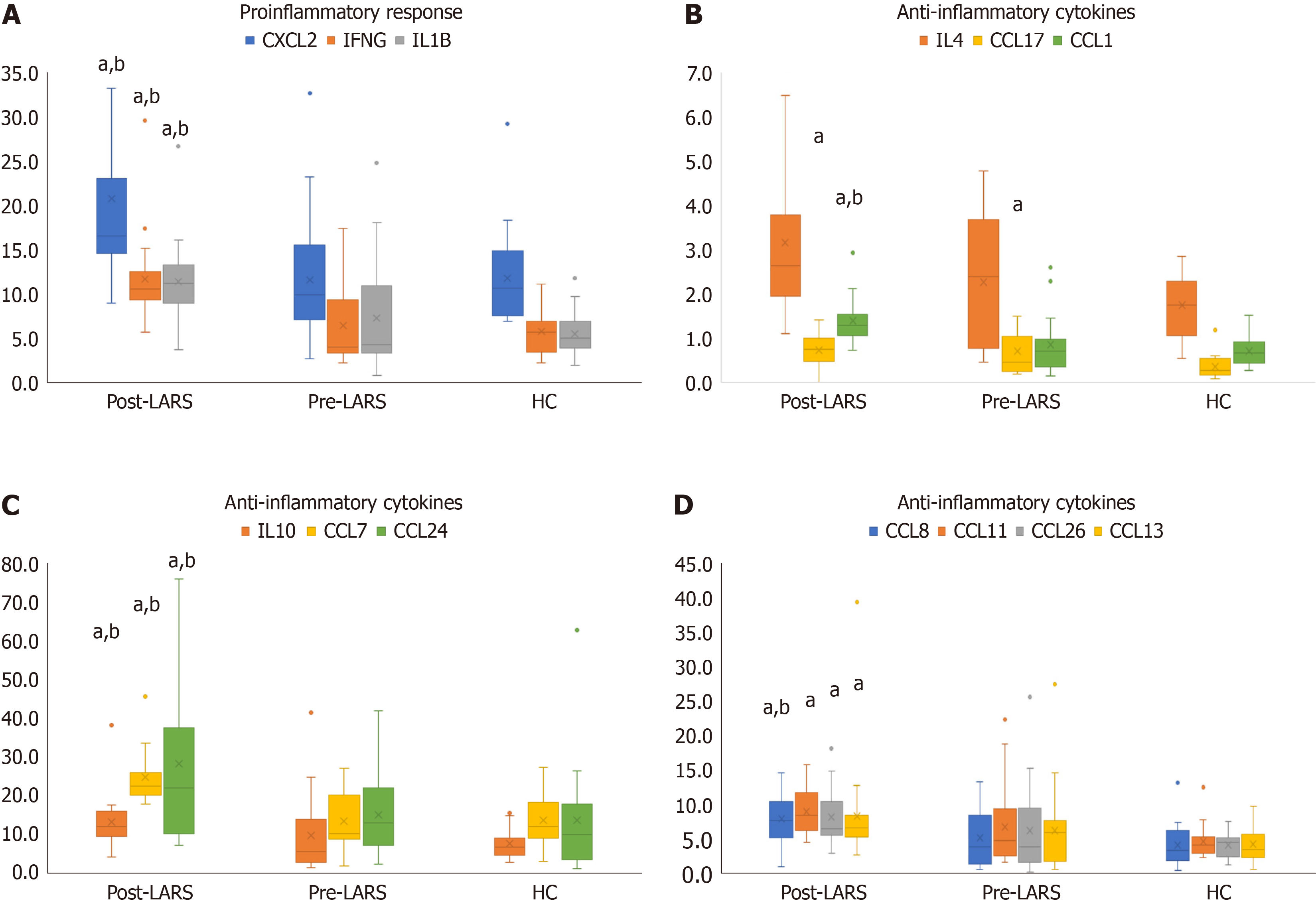
In the pre-LARS group, c-Jun levels were significantly lower compared to those in HCs (P < 0.05). Nuclear factor kappa-beta (NFκB) (P < 0.01), MEK1, and p38-MAPK levels were significantly higher than those in HCs (P < 0.05) (Table 2). The levels of the proinflammatory cytokines interleukin (IL)-1β, IFNγ and C-X-C chemokine ligand 2 (CXCL2) were signi
| Protein | mean ± SD (FI) | Median | Variance |
| MEK1 | |||
| Post-LARS | 344.9 ± 328.5 | 288.5a | 102992.8 |
| Pre-LARS | 416.3 ± 697.9 | 230 | 464957.9 |
| HC | 161.9 ± 151.4 | 102.5 | 22920.5 |
| c-Jun | |||
| Post-LARS | 48.9 ± 17.4a | 49 | 287.8 |
| Pre-LARS | 43.8 ± 10.9 | 42 | 113.6 |
| HC | 50.9 ± 11 | 50.5 | 121.1 |
| p38-MAPK | |||
| Post-LARS | 16.3 ± 15.2 | 10.0a | 219.9 |
| Pre-LARS | 12.1 ± 10.5 | 8 | 106 |
| HC | 7.3 ± 1.2 | 7 | 1.3 |
| NF-κB | |||
| Post-LARS | 10.5 ± 1.9a,b | 10.5 | 3.4 |
| Pre-LARS | 8.8 ± 3 | 8 | 8.4 |
| HC | 8.2 ± 1.6 | 8.5 | 2.6 |
Anti-inflammatory cytokines, including CCL11, CCL13, CCL17, CCL26, CCL1, CCL7, CCL8, CCL24, IL-4 and IL-10, showed a significant increase compared to levels in the HC and/or pre-LARS groups (P < 0.05) (Figure 3B-D, Supple
The levels of homeostatic cytokines, including CCL27, CCL20, CCL19, CCL23, CCL25, CXCL12 and migration in
| mean ± SD (pg/mL) | Median | Variance | ||
| CCL21 | Post-LARS | 66.8 ± 75.5 | 46.5 | 5442.8 |
| Pre-LARS | 123 ± 142.1 | 81.4a | 19274.4 | |
| HC | 48.9 ± 67.7 | 22.4 | 4588.6 | |
| CCL27 | Post-LARS | 8.5 ± 5.4b | 6.8a | 27.5 |
| Pre-LARS | 4.4 ± 4.6 | 2.1 | 20.5 | |
| HC | 3.5 ± 2.1 | 3.2 | 4.5 | |
| CCL20 | Post-LARS | 13.4 ± 17.1a | 8 | 278.8 |
| Pre-LARS | 9.4 ± 12.3 | 4.7 | 144.3 | |
| HC | 5.5 ± 4.8 | 3.1 | 22.8 | |
| CCL19 | Post-LARS | 62.2 ± 33.1a | 56.2 | 1048.1 |
| Pre-LARS | 52.9 ± 77.1 | 29.5 | 5620.6 | |
| HC | 31.8 ± 23.3 | 23.4 | 544.5 | |
| CCL23 | Post-LARS | 14.0 ± 11.6a | 10.7 | 128.5 |
| Pre-LARS | 10.8 ± 10.6 | 8.9 | 107.5 | |
| HC | 7.8 ± 4.9 | 7.7 | 24.1 | |
| CCL25 | Post-LARS | 629.7 ± 104a,b | 631.9 | 10318.7 |
| Pre-LARS | 391.2 ± 170 | 315 | 27576.1 | |
| HC | 365.7 ± 121 | 379.7 | 14629.2 | |
| CXCL12 | Post-LARS | 76.2 ± 71.8 | 52.8a | 4912 |
| Pre-LARS | 83.2 ± 147.1 | 48.6 | 20568.1 | |
| HC | 40.4 ± 20.2 | 33.8 | 408.6 | |
| MIF | Post-LARS | 167465.0 ± 61928.5a,b | 160339 | 106780256 |
| Pre-LARS | 95257.5 ± 61594.5 | 63357.2 | 362142872 | |
| HC | 89235.6 ± 51629.5 | 82917.8 | 266560193 |
GERD is typically treated with PPIs, aimed at suppressing gastric acid secretion. However, long-term drug use can pose challenges, and some patients may exhibit a low response to PPI treatment, necessitating a more permanent solution. Laparoscopic antireflux surgery is offered as an alternative method to alleviate reflux symptoms, with a success rate of approximately 90%[8,9] in experienced centers. Following LARS, the contact of the esophagus with gastric contents and noxious agents is significantly reduced, leading to a drastic alleviation of symptoms and observable healing of the epi
In this study, CCL21 levels were found to be higher in pre-LARS patients compared to controls. These intriguing findings may be explained by the role of the CCL21/CCR7 axis in the regulation of T-cell immunity. Unsoeld et al[10] observed that transgenic mice with high expression of CCL21 failed in the CD4 T-cell response against local skin in
We investigated cellular-level changes before and after surgical treatment to comprehend the pathophysiological me
Additionally, the expression level of RAF1, an activator of the MEK/ERK pathway that transmits chemical signals outside the cell to the cell nucleus, was significantly increased in post-LARS patients. Overactivity of these pathways results in NFκB activation and subsequently increased levels of pro-inflammatory cytokines, especially IL1β and IFNγ. In our study, the elevated IL1β levels may have been regulated by these two pathways and NFκB[14,15].
When we evaluated our data concerning the type of reflux, we observed varied responses in protein levels after sur
While MAPK4, TNF receptor, HRAs and AKT1 gene expression decreased, the expression of proinflammatory molecules (IL1β, IFNγ), chemotactic molecules (MIF, CCL1, CCL7, CCL11, CCL25, CCL27, CXCL2), and macrophage activation-related proteins (IL10, CCL19) increased after surgery in patients with ERD A/B and NERD compared to presurgical levels.
IL1β, a potent proinflammatory regulator, is secreted from many immune cells and triggers the production of acute phase proteins, proinflammatory cytokines, and adhesion molecules. It also activates T and B lymphocytes[16]. Together, IFNγ and TNFα are precursors of the inflammatory response. IFNγ, predominantly secreted from activated T lympho
The elevation of CXCL2 levels in post-LARS provides evidence of the presence of neutrophils in the tissue[19]. IL1β also induces the production of macrophage MIF, a regulator of innate immunity. MIF mostly causes macrophage accumulation in hypersensitivity regions[20]. MIF contributes to the activation of NFκB by inhibiting the MEK/ERK signaling pathway and IKBA, an inhibitor of NFκB[21]. It might be suggested that the TLR signaling pathway through MAP kinase and the MEK/ERK pathway was suppressed after surgery, likely due to depletion of stimulants in the lumen. On the other hand, the proinflammatory status, demonstrated by increases in IL1β, NFκB, and IFNγ levels, remained active in tissues after surgery.
This could be explained by two theories: Oxidative stress that might be elevated due to ischemia-reperfusion after surgery stimulates NFκB activation by increasing nuclear factor E2-related factor 2 and heme oxygenase levels. The second explanation involves chloride sensing regulation of NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) inflammasome activation[22,23]. Recent studies have shown that the chloride concentration in cells is a critical control point for NLRP3 inflammasome activation. Mayes-Hopfinger et al[22] revealed that decreased intracellular Cl activates the NLRP3 inflammasome, promoting an immune response by switching the proinflammatory status of a pha
TECK/CCL25 and CTACK/CCL27 levels were also increased in the post-LARS group compared to both the pre-LARS and HC groups. T memory and effector lymphocytes activated by IL1β rapidly migrate to the inflammatory epithelium via CCL25 and CCL27. However, it is known that these two chemokines primarily act via memory T cells[19]. CCL25 and CCL27 have more homeostatic effects on memory cells[19,24].
Additionally, I309/CCL1 and monocyte chemoattractant protein (MCP)2/CCL8, which have a homeostatic effect on memory T cells and have anti-inflammatory effects on Th2 and regulatory T cells during inflammation, were significantly increased after surgery. Moreover, there was an increase in MCP3/CCL7[25] and IL-10 levels, which can block the Th1 response that mediates monocyte motility, supported the anti-inflammatory activation post-LARS.
Our study showed that EOTAXIN-2/CCL24, MIP1d/CCL15, and MIP3b/CCL19 levels increased in NERD patients after surgery. EOTAXIN2/CCL24, responsible for the recruitment of basophils and eosinophils, promotes cell migration and regulates inflammatory and fibrotic activities. It is secreted from various cells, especially activated fibroblasts, leading to fibroblast proliferation and collagen synthesis[26]. The increase in CCL24 levels indicates that the collagen deposition and reorganization process was active, with the effect of anti-inflammatory regulation in post-LARS tissues. Increased EGF expression in this group also supports the healing process and proliferation[27] and provides information about the presence of eosinophils or basophils in tissues.
CC chemokines are well-known chemoattractants for monocytes (RANTES, MCP 1-5), eosinophils (eotaxins 1-3), basophils (MCP 4-5), and lymphocytes [macrophage inflammatory protein (MIP)-1α and β]. Our study demonstrated an increase in CC chemokines and many chemoattractants (MIP1, MIP3, EOTAXIN-2) after surgery in NERD patients. An increase in TNFα and IL1β might suggest that surgical treatment in NERD patients induced inflammatory processes, li
On the other hand, elevated levels of CCL1, CCL11 and CCL24 indicate that the Th2 response is activated, and that the resolution of the inflammatory response is increased post-LARS. CCL11 mediates the Th2 response as well as eosinophil and basophil migration[19]. In addition to its anti-inflammatory properties, CCL17 also helps maintain homeostatic ba
MPIF1/CCL23 levels increased after surgery in patients with ERD A/B. CCL23 secreted by neutrophils via CXCL2, supports the inflammatory response by activating lymphocytes, monocytes and macrophages[28]. However, homeostatic chemokines, such as MIP3-b/CCL19 and SDF1-a+b/CXCL12 were also increased[24] along with CCL25 and CCL27. CCL19 helps stabilize the inflammatory response by inducing naive T cells and central memory T cells to return to lymph nodes[25]. Similarly, neutrophils, monocytes and B cells return to the bone marrow and mediate the suppression of the inflammatory response[19].
IL-4 levels increased after surgery in NERD patients compared to HCs. IL-4 has potent cytoprotective properties[29]. We thought that it may have a major role in preserving the mucosal integrity after surgery. IL-4 also exerts anti-inflammatory effects by inducing the production of CCL7 and CCL11 from peripheral cells in the inflammatory region[25]. These two elevated chemokines may be secreted via IL-4. IL-4 can also suppress important cytokines in the proinflammatory process, such as IL1β and TNFα[30,31]. The significant increase in important anti-inflammatory cytokines such as IL-4 and IL-10 in the NERD and ERD A/B groups may have caused the suppression of important proinflammatory markers in the postoperative group[32].
These findings suggest that the postoperative recovery process is ongoing after successful surgery. In addition, the proinflammatory effect is still ongoing, and it is possible that the anti-inflammatory response overwhelms the ongoing proinflammatory process. After the operation, patients were rescored for symptoms, improvements noted in the control endoscopy, and relapsed patients were excluded. But there may be patients whose mucosal damage had healed but who still had insensible acid attacks. Therefore, reflux symptoms that may occur after LARS may not always indicate failure of the surgery. A limitation of our study is the inability to perform a 24-h pH-impedance test in the post-LARS group, pre
In conclusion, inflammatory processes, especially involving the TLR signaling pathway, may play a significant role in the pathophysiology of reflux disease. Surgical treatment of reflux disease yields varied responses in cells: The MEK/ERK pathway is suppressed, while inflammatory molecule levels, particularly NFκB and IL1β, increase through different me
The post-LARS group was included in the study approximately 6 months (2-18 months) after the operations. Proin
Laparoscopic anti-reflux surgery (LARS) is the preferred therapeutic approach for gastroesophageal reflux disease (GERD), as it effectively prevents the reflux of gastric contents into the esophagus. While there is existing knowledge about the recovery period of LARS (typically reported as 8-10 wk in the literature), limited data is available regarding the healing process within the esophageal mucosa following this procedure. This study aims to illuminate the recovery process of patients with GERD who have undergone LARS, with a specific focus on the inflammatory pathways within the esophageal mucosa.
Patients who have undergone LARS often report the eventual healing of symptoms such as heartburn and regurgitation after the surgery. However, a small percentage continues to experience GERD symptoms even post-LARS. The available data on LARS is primarily derived from patients’ responses.
We aim to focus on the inflammatory and recovery processes within the esophageal mucosa before and after the surgery.
Twenty-two patients with GERD (the same patients before and after LARS) and 25 healthy controls (HCs) were enrolled in the study. Esophageal biopsies were homogenized, and the expressions of inflammatory and cell signaling genes were measured using real-time polymerase chain reaction. Protein levels were assessed using the multiplex enzyme-linked immunosorbent assay method.
The approximate period between pre- and post-LARS was 6 months (5.8 ± 3.8 months). We demonstrated that proinflammatory cytokines remained activated in post-LARS patients. However, we also observed a significant increase in ho
We conclude that the toll-like receptor signal is involved in the activation of inflammatory cytokines, while the MEK/ERK pathway is suppressed after LARS. Despite the higher levels of inflammatory cytokines, regulatory and anti-inflammatory markers were also activated in these patients. The persistence of cytokine levels suggests that recovery may not be complete even at 6 months. Patients who have undergone LARS should avoid refluxogenic foods to prevent short-term GERD symptoms.
We plan a follow-up study with esophageal biopsies and 24-h multichannel intraluminal impedance-pH impedance mo
The results of this study were presented and awarded with a travel grant at IUBMB-FEBS-PABMB 2022 Congress bet
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Turkish Society of Gastroenterology; Turkish Biochemical Society.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bordin DS, Russia S-Editor: Wang JJ L-Editor: A P-Editor: Xu ZH
| 1. | Gorgulu V, Ergun P, Kipcak S, Doganavsargil B, Sifrim D, Bor S. Revisiting the Role of Esophageal Mucosal Dilated Intercellular Spaces in the Diagnosis and Pathophysiology of Heartburn. J Neurogastroenterol Motil. 2023;29:436-445. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Ergun P, Kipcak S, Gunel NS, Bor S, Sozmen EY. Roles of Cytokines in Pathological and Physiological Gastroesophageal Reflux Exposure. J Neurogastroenterol Motil. 2023;. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Souza RF, Huo X, Mittal V, Schuler CM, Carmack SW, Zhang HY, Zhang X, Yu C, Hormi-Carver K, Genta RM, Spechler SJ. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology. 2009;137:1776-1784. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Ergun P, Kipcak S, Bor S. Epigenetic Alterations from Barrett's Esophagus to Esophageal Adenocarcinoma. Int J Mol Sci. 2023;24. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Dean BB, Gano AD Jr, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656-664. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Pessaux P, Arnaud JP, Delattre JF, Meyer C, Baulieux J, Mosnier H. Laparoscopic antireflux surgery: five-year results and beyond in 1340 patients. Arch Surg. 2005;140:946-951. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] [Cited in This Article: ] |
| 8. | Palmer K. Review article: indications for anti-reflux surgery and endoscopic anti-reflux procedures. Aliment Pharmacol Ther. 2004;20 Suppl 8:32-35. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Roark R, Sydor M, Chatila AT, Umar S, Guerra R, Bilal M, Guturu P. Management of gastroesophageal reflux disease. Dis Mon. 2020;66:100849. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Unsoeld H, Mueller K, Schleicher U, Bogdan C, Zwirner J, Voehringer D, Pircher H. Abrogation of CCL21 chemokine function by transgenic over-expression impairs T cell immunity to local infections. Int Immunol. 2007;19:1281-1289. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285:2944-2971. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Bahar ME, Kim HJ, Kim DR. Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Signal Transduct Target Ther. 2023;8:455. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Ahmadi A, Ahrari S, Salimian J, Salehi Z, Karimi M, Emamvirdizadeh A, Jamalkandi SA, Ghanei M. p38 MAPK signaling in chronic obstructive pulmonary disease pathogenesis and inhibitor therapeutics. Cell Commun Signal. 2023;21:314. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Wang P, Qian H, Xiao M, Lv J. Role of signal transduction pathways in IL-1β-induced apoptosis: Pathological and therapeutic aspects. Immun Inflamm Dis. 2023;11:e762. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Laurindo LF, Santos AROD, Carvalho ACA, Bechara MD, Guiguer EL, Goulart RA, Vargas Sinatora R, Araújo AC, Barbalho SM. Phytochemicals and Regulation of NF-kB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects. Metabolites. 2023;13. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Germolec DR, Shipkowski KA, Frawley RP, Evans E. Markers of Inflammation. Methods Mol Biol. 2018;1803:57-79. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Tau G, Rothman P. Biologic functions of the IFN-gamma receptors. Allergy. 1999;54:1233-1251. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Masters SL, Mielke LA, Cornish AL, Sutton CE, O'Donnell J, Cengia LH, Roberts AW, Wicks IP, Mills KH, Croker BA. Regulation of interleukin-1beta by interferon-gamma is species specific, limited by suppressor of cytokine signalling 1 and influences interleukin-17 production. EMBO Rep. 2010;11:640-646. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659-702. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Ndreu L, Sasse S, Karlberg AT, Karlsson I. Haptenation of Macrophage Migration Inhibitory Factor: A Potential Biomarker for Contact Hypersensitivity. Front Toxicol. 2022;4:856614. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | He J, Zheng L, Li X, Huang F, Hu S, Chen L, Jiang M, Lin X, Jiang H, Zeng Y, Ye T, Lin D, Liu Q, Xu J, Chen K. Obacunone targets macrophage migration inhibitory factor (MIF) to impede osteoclastogenesis and alleviate ovariectomy-induced bone loss. J Adv Res. 2023;53:235-248. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Mayes-Hopfinger L, Enache A, Xie J, Huang CL, Köchl R, Tybulewicz VLJ, Fernandes-Alnemri T, Alnemri ES. Chloride sensing by WNK1 regulates NLRP3 inflammasome activation and pyroptosis. Nat Commun. 2021;12:4546. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Perry JSA, Morioka S, Medina CB, Iker Etchegaray J, Barron B, Raymond MH, Lucas CD, Onengut-Gumuscu S, Delpire E, Ravichandran KS. Interpreting an apoptotic corpse as anti-inflammatory involves a chloride sensing pathway. Nat Cell Biol. 2019;21:1532-1543. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Palomino DC, Marti LC. Chemokines and immunity. Einstein (Sao Paulo). 2015;13:469-473. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Murphy PM, Rich RR, Fleisher TA, Shearer WT, Schroeder HW, Frew AJ, Weyand CM. Clinical Immunology. United States: Elsevier, 2018. [Cited in This Article: ] |
| 26. | Segal-Salto M, Barashi N, Katav A, Edelshtein V, Aharon A, Hashmueli S, George J, Maor Y, Pinzani M, Haberman D, Hall A, Friedman S, Mor A. A blocking monoclonal antibody to CCL24 alleviates liver fibrosis and inflammation in experimental models of liver damage. JHEP Rep. 2020;2:100064. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Villarreal-Ponce A, Tiruneh MW, Lee J, Guerrero-Juarez CF, Kuhn J, David JA, Dammeyer K, Mc Kell R, Kwong J, Rabbani PS, Nie Q, Ceradini DJ. Keratinocyte-Macrophage Crosstalk by the Nrf2/Ccl2/EGF Signaling Axis Orchestrates Tissue Repair. Cell Rep. 2020;33:108417. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Bonaventura A, Montecucco F. CCL23 is a promising biomarker of injury in patients with ischaemic stroke. J Intern Med. 2018;283:476-478. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, Saeed IT, Burnham WR, Farthing MJ. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut. 2002;50:451-459. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the Anti-Inflammatory Cytokines Interleukin-4 and Interleukin-10 during Pregnancy. Front Immunol. 2014;5:253. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Dunleavy C, Elsworthy RJ, Upthegrove R, Wood SJ, Aldred S. Inflammation in first-episode psychosis: The contribution of inflammatory biomarkers to the emergence of negative symptoms, a systematic review and meta-analysis. Acta Psychiatr Scand. 2022;146:6-20. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679-692. [PubMed] [DOI] [Cited in This Article: ] |