Published online Mar 27, 2024. doi: 10.4240/wjgs.v16.i3.790
Peer-review started: December 22, 2023
First decision: January 9, 2024
Revised: January 21, 2024
Accepted: February 29, 2024
Article in press: February 29, 2024
Published online: March 27, 2024
Upper gastrointestinal bleeding (UGIB) is a common medical emergency and early assessment of its outcomes is vital for treatment decisions.
To develop a new scoring system to predict its prognosis.
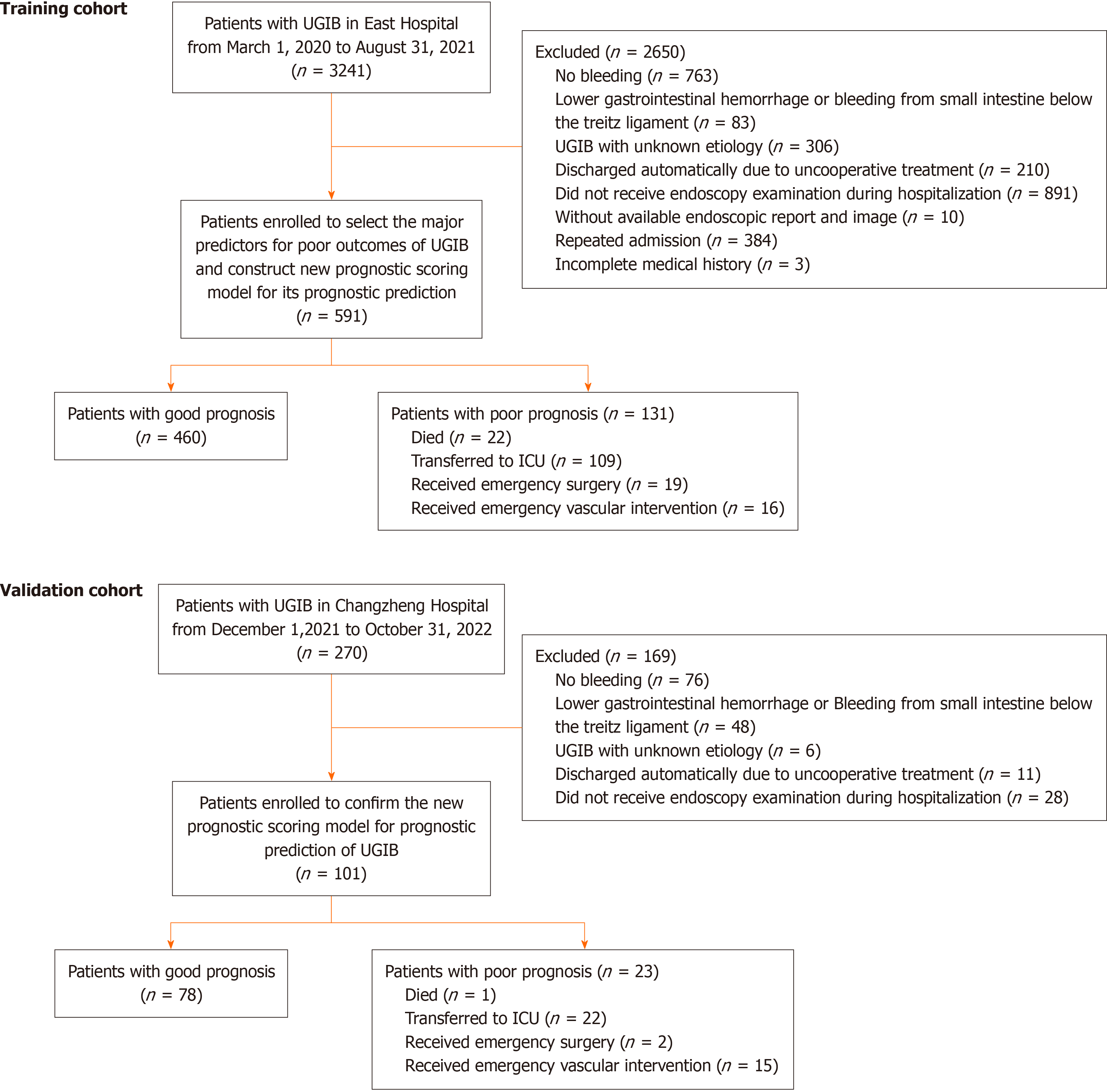
In this retrospective study, 692 patients with UGIB were enrolled from two cen
Totally 22.2% (131/591) patients in the training cohort and 22.8% (23/101) in the validation cohort presented poor outcomes. Based on the stepwise-forward Lo
The MH-STRALP score and pre-MH-STRALP score are simple, convenient, and accurate tools for prognosis prediction of UGIB, and may be applied for early decision on its management strategies.
Core Tip: This study carried out a retrospective study to develop new scoring systems to predict the prognosis of upper gastrointestinal bleeding (UGIB). The patients with UGIB in two centers were enrolled into a training cohort (n = 591) and a validation cohort (n = 101). A new post-endoscopic prognostic scoring system (MH-STRALP) and a pre-endoscopic model were conducted and determined with nomograms. The two scores showed better predictive value in both training cohort and validation cohort than the other scores. Thus, we believe that we provided simple, convenient, and accurate tools for UGIB prognostication and early decision on the management strategies.
- Citation: Hu JN, Xu F, Hao YR, Sun CY, Wu KM, Lin Y, Zhong L, Zeng X. MH-STRALP: A scoring system for prognostication in patients with upper gastrointestinal bleeding. World J Gastrointest Surg 2024; 16(3): 790-806
- URL: https://www.wjgnet.com/1948-9366/full/v16/i3/790.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i3.790
Upper gastrointestinal bleeding (UGIB) is a type of bleeding that originates above the Treitz ligament and always results from an esophageal, gastroduodenal, and biliary or pancreatic disease involving the duodenum[1]. According to the cause of bleeding, it is divided into two categories: Nonvariceal UGIB (NVUGIB) and variceal UGIB (VUGIB). The com
As we all know, the greatest risk of UGIB is to lead to life-threatening peripheral circulatory failure. Although the glo
It has been well documented that a precise risk assessment is crucial to aid clinical decision-making and guide subse
With this study, we aimed to investigate the main factors influencing the poor outcomes of UGIB (e.g., the demand for emergency surgery or vascular intervention, being transferred to the intensive care unit (ICU), and dying during hospitalization) and attem
This is a multicenter, retrospective, cohort study. All procedures were performed in accordance with the ethical standards of the Responsible Committee on Human Experimentation (institutional and national) and the 1975 Helsinki Declaration, as revised in 2008. The study protocol was reviewed and approved by the Institutional Ethics Committee of Shanghai Changzheng Hospital (2016SL018).
To determine the impact factors of the outcomes of UGIB and to develop the prognostic scoring system, UGIB patients admitted in East Hospital from March 1, 2020 to August 31, 2021 were enrolled into a training cohort; patients admitted in Changzheng hospital from December 1, 2021 to October 31, 2022 were enrolled into a validation cohort. The eligibility criteria were as follows: (1) Diagnosis of UGIB by gastrointestinal endoscopy, capsule endoscopy, or enteroscopy; (2) with a clear etiology of UGIB at discharge; and (3) with sufficient clinical information, including medical history, clinical manifestations, physical signs, endoscopic findings, and laboratory examination results. The exclusion criteria were as follows: (1) Unexplained bleeding (e.g., the location or cause could not be identified even if endoscopy and various exa
In this retrospective study, the data of the individuals in the training cohort were used to select the major predictors for poor outcomes and to construct a new post-endoscopic prognostic scoring model involving endoscopic signs. Sequentially, a nomogram was determined to present the model and was compared with the previous prognostic scoring sys
The clinical data were collected using a database conducted by Epidata 3.1, including medical history, symptoms, vital and other physical signs, initial laboratory findings after admission, endoscopic characteristics [e.g., dark spots, active bleeding, adherent thrombosis, visible vessels, lesions with diameter ≥ 2 cm, stigmata of recent hemorrhage (SRH)], main treatment strategies (e.g., the use of proton pump inhibitor, somatostatin and its analogs, blood transfusion, endoscopic management, vascular intervention, and emergency surgery) and the outcomes.
The primary endpoint was the compound outcomes, defined as (1) the demand for emergency surgery or vascular inter
Categorical variables were expressed as numbers and percentages or frequencies. Continuous parameters were described using medians with interquartile ranges. The χ2 test or Fisher’s exact test was used to compare the categorical variables. The comparison of continuous variables was determined by the t-test or Mann-Whitney U-test, depending on whether these variables were from a normally distributed aggregate and were consistent with the homogeneity of variance. Uni
All authors had access to the study data and reviewed and approved the final manuscript. The corresponding authors made the final decision to submit the manuscript for publication.
Patients with UGIB admitted in two centers were enrolled in this study. All patients had a clear UGIB etiology at dis
| GP group (n = 460) | PP group (n = 131) | P value | |
| Sex | 0.710 | ||
| Male, n (%) | 351 (76.3) | 102 (77.9) | |
| Female, n (%) | 109 (23.7) | 29 (22.1) | |
| Age (yr), median (IQR) | 63.0 (21.0) | 65.0 (16.0) | 0.020 |
| Height (cm), median (IQR) | 170.0 (11.0) | 170.0 (11.0) | 0.126 |
| Weight (kg), median (IQR) | 70.0 (15.3) | 65.0 (20.0) | 0.006 |
| BMI (kg/m2), median (IQR) | 22.0 (5.0) | 21.0 (7.0) | 0.008 |
| Smoke, n (%) | 130 (28.3) | 46 (35.1) | 0.089 |
| Drink, n (%) | 76 (16.5) | 23 (17.6) | 0.717 |
| Previous UGIB history, n (%) | 125 (27.2) | 48 (36.6) | 0.036 |
| Etiology, n (%) | |||
| Peptic ulcer | 323 (72.2) | 47 (35.9) | < 0.001 |
| EGVB | 43 (9.3) | 47 (35.9) | < 0.001 |
| UGIC | 29 (6.3) | 23 (17.5) | < 0.001 |
| Acute erosive hemorrhagic gastritis | 30 (6.5) | 4 (3.1) | 0.197 |
| Mallory-Weiss syndrome | 17 (3.7) | 2 (1.5) | 0.337 |
| Dieulafoy’s disease | 6 (1.3) | 2 (1.5) | 1.000 |
| Bleeding due to endoscopic operations | 2 (0.4) | 1 (0.8) | 1.000 |
| Biliary bleeding | 0 | 2 (1.5) | 0.049 |
| Pancreatic bleeding | 0 | 1 (0.8) | 0.222 |
| Polyp bleeding | 1 (0.2) | 0 | 1.000 |
| Comorbidities, n (%) | |||
| Hypertension | 202 (43.9) | 64 (48.9) | 0.316 |
| Diabetes | 108 (23.5) | 39 (29.8) | 0.142 |
| Coronary atherosclerotic heart disease | 81 (17.6) | 21 (16.0) | 0.673 |
| Chronic liver disease | 71 (15.4) | 43 (32.8) | < 0.001 |
| Liver cirrhosis | 48 (10.4) | 44 (33.6) | < 0.001 |
| Respiratory disease | 22 (4.9) | 11 (8.4) | 0.112 |
| Gallstones | 15 (3.3%) | 11 (8.4%) | 0.011 |
| Chronic kidney disease | 26 (5.7) | 9 (6.9) | 0.602 |
| Hematologic disease | 2 (0.4) | 0 | 1.000 |
| Autoimmune disease | 3 (0.7) | 4 (3.1) | 0.074 |
| Cerebral infarction | 46 (10.0) | 25 (19.1) | 0.005 |
| Stroke | 50 (10.9) | 26 (19.8) | 0.007 |
| Malignancy | 49 (10.7) | 42 (32.1) | < 0.001 |
| Multi-organ failure | 8 (1.7) | 10 (7.6) | 0.001 |
| Heart failure | 89 (19.3) | 26 (19.8) | 0.899 |
| Liver failure | 46 (10.0) | 47 (35.9) | < 0.001 |
| Renal failure | 24 (5.2) | 9 (6.9) | 0.467 |
| Respiratory failure | 0 (0.0) | 2 (1.5) | 0.049 |
| Helicobacter pylori infection, n (%) | 155 (33.7) | 22 (16.8) | 0.490 |
| Drugs, n (%) | |||
| Antiplatelet drugs | 107 (23.3) | 28 (21.4) | 0.641 |
| Anticoagulants | 5 (1.1) | 3 (2.3) | 0.533 |
| Glucocorticoids | 1 (0.2) | 0 | 1.000 |
| NSAIDS | 5 (1.1) | 4 (3.1) | 0.224 |
| Operation, n (%) | |||
| Surgery | 30 (6.5) | 15 (11.5) | 0.061 |
| EMR or ESD | 4 (0.9) | 0 | 0.580 |
| Time from symptom onset to admission (h), median (IQR) | 24.0 (65.0) | 12.0 (70.0) | 0.033 |
| Symptoms at admission, n (%) | |||
| Haematemesis | 184 (40.0) | 90 (68.7) | < 0.001 |
| Black stool | 367 (79.8) | 89 (67.9) | 0.004 |
| Abdominal pain | 156 (33.9) | 41 (31.3) | 0.575 |
| Palpitations | 192 (41.7) | 89 (67.9) | < 0.001 |
| Amaurosis | 40 (8.7) | 20 (15.3) | 0.028 |
| Syncope | 38 (8.3) | 15 (11.5) | 0.260 |
| Sweat | 94 (20.4) | 44 (33.6) | 0.002 |
| Altered mental status | 3 (0.7) | 4 (3.1) | 0.074 |
| Body signs at admission | |||
| Pulse, median (IQR) | 84.0 (13.8) | 95.0 (27.0) | < 0.001 |
| SBP, median (IQR) | 124.0 (22.0) | 120.0 (30.0) | 0.011 |
| DBP, median (IQR) | 74.0 (14.0) | 68.0 (16.0) | < 0.001 |
| Anemia appearance, n (%) | 201 (43.7) | 99 (75.6) | < 0.001 |
| Abdominal tenderness, n (%) | 63 (13.7) | 23 (17.6) | 0.269 |
| Laboratory findings at admission | |||
| RBC, ×1012/L, median (IQR) | 3.0 (1.0) | 3.0 (1.0) | < 0.001 |
| WBC, ×109/L, median (IQR) | 9.0 (4.0) | 9.0 (6.0) | 0.952 |
| Neutrophil, ×109/L, median (IQR) | 6.0 (5.0) | 6.0 (6.0) | 0.141 |
| Lymphocyte, ×109/L, median (IQR) | 2.0 (1.00) | 1.0 (1.0) | < 0.001 |
| MCV (fl), median (IQR) | 92.0 (7.0) | 90.0 (12.0) | 0.012 |
| HCT, median (IQR) | 31.0 (12.0) | 25.0 (11.0) | < 0.001 |
| Hemoglobin (g/L), median (IQR) | 104.0 (43.0) | 81.0 (39.0) | < 0.001 |
| PLT (×109/L), median (IQR) | 215.0 (107.0) | 174.0 (153.0) | < 0.001 |
| Reticulocyte (×109/L), median (IQR) | 89.0 (60.0) | 110.0 (92.0) | 0.219 |
| CRP, median (IQR) | 2.0 (4.0) | 3.0 (16.0) | < 0.001 |
| PT (s), median (IQR) | 12.0 (1.0) | 13.0 (3.0) | < 0.001 |
| INR, median (IQR) | 1.0 (0) | 1.0 (0) | 0.002 |
| Fibrinogen (g/L), median (IQR) | 2.0 (1.0) | 2.0 (1.0) | 0.002 |
| D-dimer (mg/L), median (IQR) | 0 (1.0) | 1.0 (3.0) | < 0.001 |
| Albumin (g/L), median (IQR) | 37.0 (8.0) | 30.0 (8.0) | < 0.001 |
| Urea (mmol/L), median (IQR) | 9.0 (8.0) | 10.0 (10.0) | 0.250 |
| Creatinine (μmol/L), median (IQR) | 79.0 (31.0) | 75.0 (37.0) | 0.040 |
| Serum iron (μmol/L), median (IQR) | 10.0 (12.0) | 8.0 (9.0) | 0.030 |
| Ferritin (μg/L), median (IQR) | 107.5 (188.5) | 115.0 (253.5) | 0.947 |
| Timing of endoscopy after admission (d), median (IQR) | 36.0 (56.0) | 12.0 (31.0) | < 0.001 |
| Endoscopic appearance, n (%) | |||
| Dark spots | 25 (5.4) | 14 (10.7) | 0.033 |
| Bleeding | 80 (17.4) | 61 (46.6) | < 0.001 |
| Adherent thrombosis | 144 (31.3) | 91 (69.5) | < 0.001 |
| Visible vessels | 97 (21.1) | 68 (51.9) | < 0.001 |
| Lesion diameter ≥ 2 cm | 54 (11.7) | 25 (19.1) | 0.001 |
| SRH | 192 (41.7) | 109 (83.2) | < 0.001 |
| Treatment, n (%) | |||
| Blood transfusion | 74 (16.1) | 92 (70.2) | < 0.001 |
| Proton pump inhibitor | 459 (99.8) | 131 (100.0) | 1.000 |
| Somatostatin and its analogs | 328 (71.3) | 121 (92.4) | < 0.001 |
| Endoscopic treatment | 110 (23.9) | 75 (57.3) | < 0.001 |
| Local injection of Adrenaline | 17 (3.7) | 4 (3.1) | 0.934 |
| Thermocoagulation | 47 (10.2) | 23 (17.6) | 0.022 |
| Local injection of a sclerosing agent | 25 (5.4) | 35 (26.7) | < 0.001 |
| Ligation | 24 (5.2) | 33 (25.2) | < 0.001 |
| Titanium clip | 39 (8.5) | 24 (18.3) | 0.001 |
| Rebleeding, n (%) | 15 (3.3) | 26 (19.8) | < 0.001 |
According to the previously identified compound outcomes, the patients were divided into a PP and a good prognosis (GP) group. In the training cohort, compared with those in the GP group, the PP patients were older (P = 0.020) and had lower body weight (P =0.006) and body mass index (BMI, P = 0.008). Totally, 36.6% of patients in the PP group had pre
The data of the cases in the training cohort were used to determine the impact factors of the prognosis and to establish a new scoring system for UGIB prognostication. The prognostic factors of UGIB were determined by univariate and mul
| Univariate Logistic analysis | Multivariate Logistic stepwise forward analysis | ||||||
| OR | P value | 95%CI | OR | z value | P value | 95%CI | |
| Age | 1.017 | 0.010 | 1.004-1.030 | ||||
| Weight | 0.974 | 0.004 | 0.957-0.992 | ||||
| BMI | 0.916 | 0.002 | 0.866-0.968 | ||||
| Previous UGIB history | 1.550 | 0.036 | 1.028-2.337 | ||||
| Liver disease | 2.677 | < 0.001 | 1.718-4.173 | ||||
| Cirrhosis | 4.341 | < 0.001 | 2.713-6.945 | ||||
| Autoimmune diseases | 4.798 | 0.042 | 1.060-21.715 | ||||
| Cerebral infarction | 2.123 | 0.006 | 1.247-3.612 | ||||
| Stroke | 2.030 | 0.008 | 1.207-3.416 | 1.710 | 1.45 | 0.148 | 0.826-3.540 |
| Liver failure | 5.036 | < 0.001 | 3.149-8.052 | 2.370 | 2.66 | 0.008 | 1.254-4.480 |
| Hematemesis | 3.293 | < 0.001 | 2.177-4.980 | ||||
| Black stool | 0.537 | 0.005 | 0.349-0.827 | ||||
| Palpitation | 2.958 | < 0.001 | 1.961-4.462 | ||||
| Amaurosis | 1.892 | 0.030 | 1.063-3.366 | ||||
| Sweat | 1.969 | 0.002 | 1.284-3.020 | ||||
| Altered mental status | 4.798 | 0.042 | 1.060-21.715 | ||||
| Systolic blood pressure | 0.986 | 0.006 | 0.976-0.996 | ||||
| Diastolic blood pressure | 0.959 | < 0.001 | 0.942-0.976 | ||||
| Pulse | 1.044 | < 0.001 | 1.031-1.057 | 1.039 | 4.73 | < 0.001 | 1.023-1.055 |
| Anemia appearance | 3.971 | < 0.001 | 2.560-6.161 | ||||
| Neutrophil count | 1.061 | 0.010 | 1.014-1.110 | ||||
| Lymphocyte count | 0.647 | < 0.001 | 0.509-0.823 | ||||
| RBC | 0.436 | < 0.001 | 0.341-0.557 | ||||
| MCV | 0.969 | 0.007 | 0.947-0.991 | ||||
| HCT | 0.901 | < 0.001 | 0.876-0.927 | ||||
| Hemoglobin | 0.972 | < 0.001 | 0.964-0.980 | ||||
| CRP | 1.009 | 0.007 | 1.002-1.015 | ||||
| Fibrinogen | 0.768 | 0.013 | 0.624-0.947 | ||||
| D-dimer | 1.081 | 0.006 | 1.023-1.142 | ||||
| Albumin | 0.814 | < 0.001 | 0.780-0.851 | 0.912 | -3.37 | 0.001 | 0.864-0.962 |
| Ferritin | 1.001 | 0.004 | 1.000-1.001 | ||||
| Blood transfusion | 12.305 | < 0.001 | 7.849-19.290 | 4.387 | 5.00 | < 0.001 | 2.458-7.831 |
| Somatostatin and its analogs | 4.870 | < 0.001 | 2.477-9.572 | ||||
| Peptic ulcer | 0.225 | < 0.001 | 0.150-0.339 | ||||
| UGIC | 3.165 | < 0.001 | 1.761-5.690 | 3.795 | 3.32 | 0.001 | 1.727-8.337 |
| EGVB | 5.426 | < 0.001 | 3.373-8.729 | ||||
| Dark spots | 2.082 | 0.036 | 1.049-4.132 | ||||
| Bleeding | 4.139 | < 0.001 | 2.721-6.297 | ||||
| Adherent thrombosis | 4.992 | < 0.001 | 3.278-7.604 | ||||
| Visible vessels | 4.039 | < 0.001 | 2.682-6.083 | ||||
| Lesion diameter ≥ 2 cm | 2.609 | 0.001 | 1.492-4.559 | ||||
| SRH | 6.916 | < 0.001 | 4.219-11.336 | 4.089 | 4.43 | <0.001 | 2.192-7.626 |
| Rebleeding | 7.417 | < 0.001 | 3.794-14.500 | 2.071 | 1.69 | 0.090 | 0.892-4.809 |
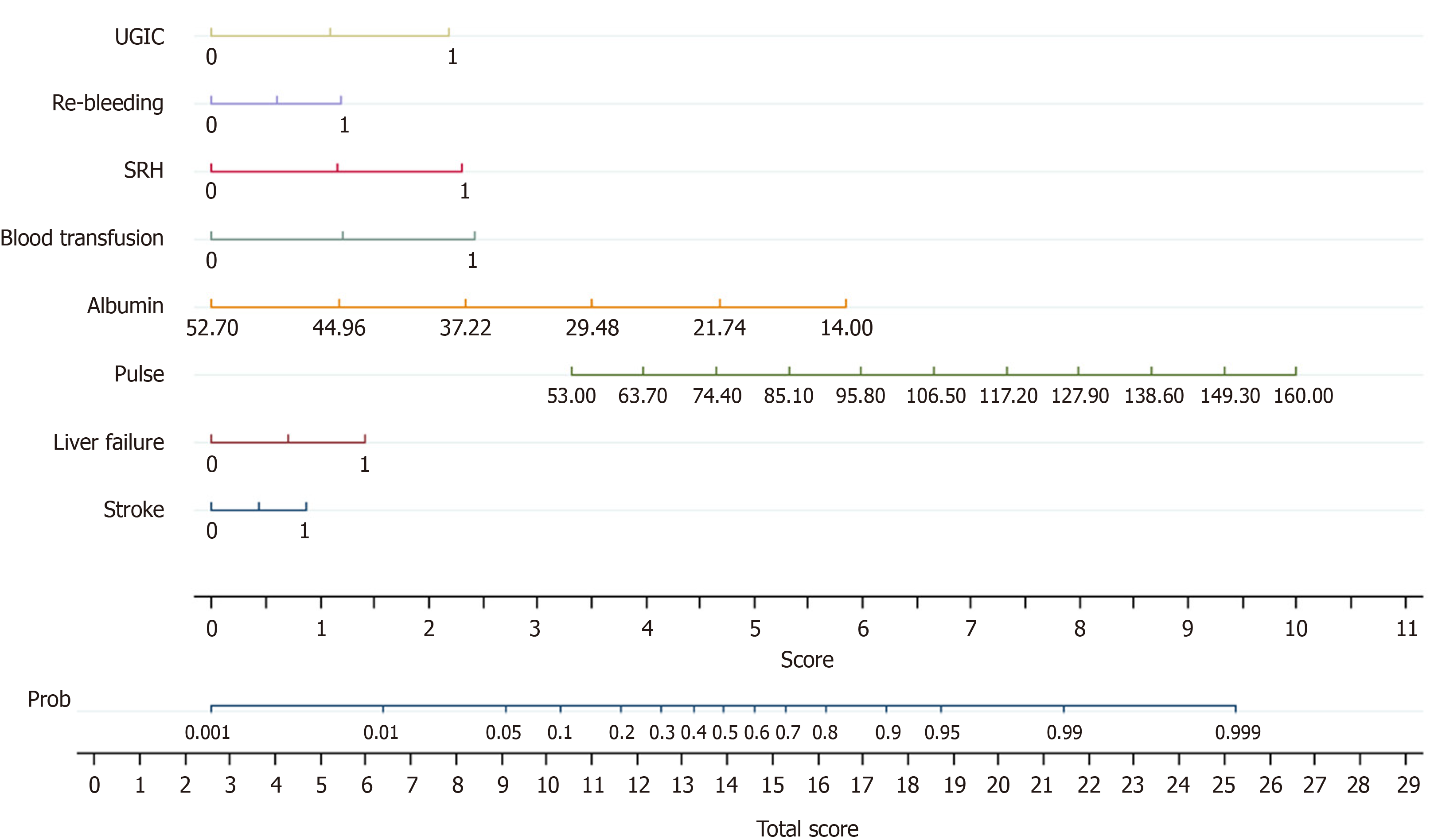
A nomogram was determined to present the post-endoscopic prognostic model, MH-STRALP (Figure 2). In the nomo
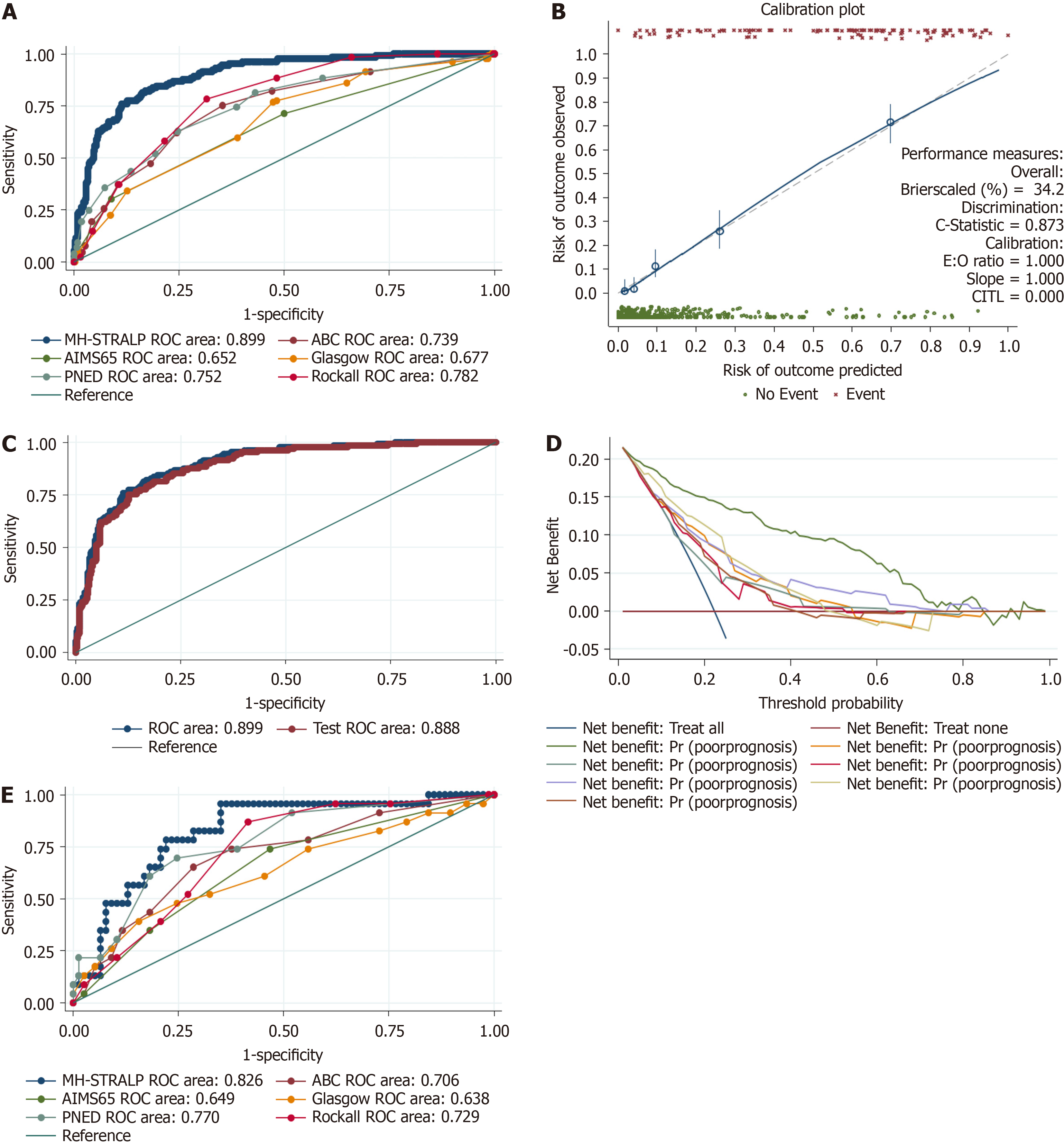
| Scoring system | AUROC (95%CI) |
| MH-STRALP | 0.899 (0.870-0.928) |
| ABC | 0.739 (0.691-0.788) |
| AIMS65 | 0.652 (0.599-0.704) |
| Glasgow | 0.677 (0.626-0.727) |
| PNED | 0.752 (0.704-0.800) |
| Rockall | 0.782 (0.743-0.822) |
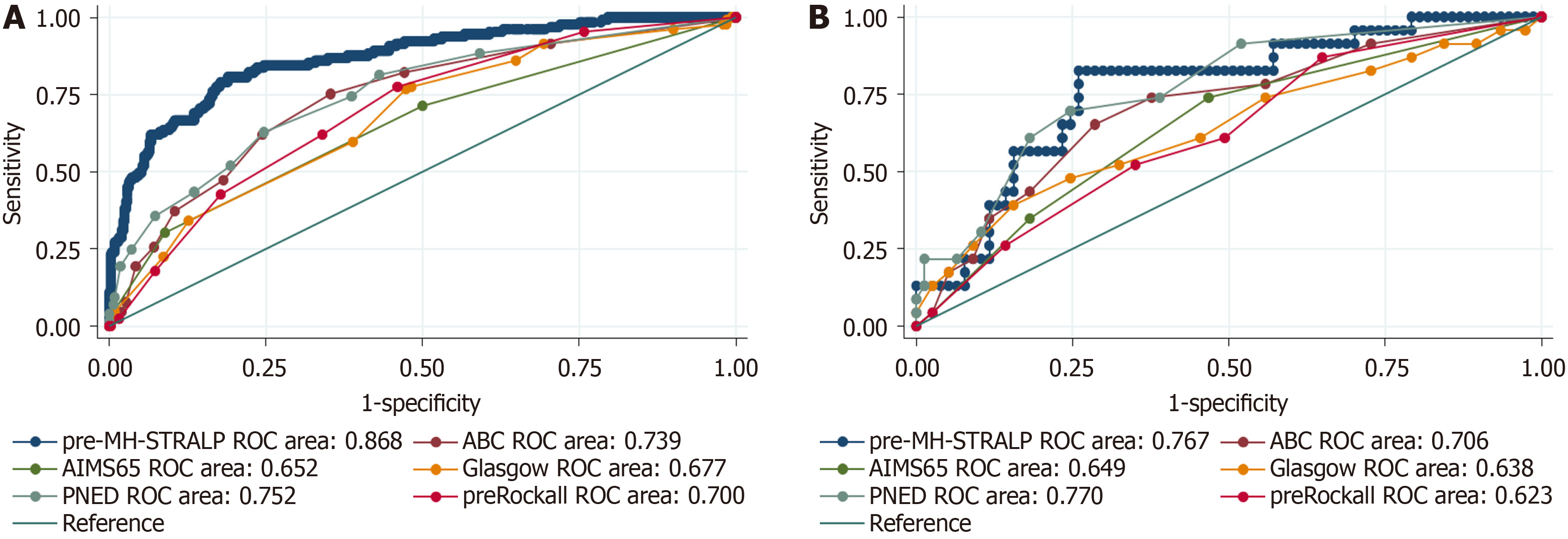
Some patients were unable to undergo endoscopy during hospitalization due to various contraindications; therefore, we conducted a new pre-endoscopic model (pre-MH-STRALP score) by removing the endoscopic indicators. Six factors (i.e., pulse, albumin, history of liver failure or stroke, demand for blood transfusion, and rebleeding) were included in the pre-MH-STRALP score. The AUROCs of the new pre-endoscopic model for discriminating the UGIB prognosis were 0.868 (95%CI: 0.832-0.904) in the training cohort and 0.767 (95%CI: 0.832 to 0.904) in the validation cohort, thereby representing better predictive performance than other pre-endoscopic scoring systems (Figure 4, Table 4, Supplementary Table 3).
| Scoring system | AUROC (95%CI) |
| pre-MH-STRALP | 0.868 (0.832-0.904) |
| ABC | 0.739 (0.691-0.788) |
| AIMS65 | 0.652 (0.599-0.704) |
| Glasgow | 0.677 (0.626-0.727) |
| PNED | 0.752 (0.704-0.800) |
| pre-Rockall | 0.700 (0.652-0.748) |
UGIB is a common emergency and remains challenging to treat and manage. Multiple factors can contribute to the deterioration of the patients' condition and ultimately lead to a PP. To date, drug administrations and emergency endoscopy remain as the standard treatment regimens for UGIB management, as recommended by a series of guidelines[9-12,23,24]. However, despite administration of high-dose proton pump inhibitors and other drugs as well as repeated intervention of endoscopic hemostasis, there were still some patients who needed transcatheter embolization or surgical treatment. Several models have already been suggested for UGIB prognostication. Nevertheless, only few studies focused on the compound outcomes, which not only led to poorer survival but also prolonged the length of hospital stay and enhanced medical burden. With this study, we constructed a new scoring system, MH-STRALP, to predict the prognosis of patients with UGIB, with compound endpoint events of undergoing emergency surgery or vascular intervention, being trans
Among all the etiologies, UGIC and liver cirrhosis have been regarded as relevant to poor UGIB prognosis. UGIC is the third leading cause of UGIB in addition to ulcers and varices, accounting for 3.7%-5% of UGIB patients[25-27]. For 79% of patients with UGIC, UGIB is the initial manifestation of cancer[26]. Previous studies shown that the prognosis for UGIC is extremely poor, with a median survival of 1.3-3.0 months after bleeding due to advanced gastric cancer[25,27,28]. Because of the severity of the protopathy diseases as well as the difficulty of endoscopic and vascular intervention, it is generally agreed that VUGIB resulting from liver cirrhosis-related hypertension is more rapid and serious as compared with N
Some underlying diseases, such as stroke, have been implicated in UGIB prognosis. Stroke, including hemorrhagic and ischemic stroke, is one of the important predispositions of stress ulcer-induced UGIB[34-36]. According to an investiga
Circulatory failure is the most important manifestation and serious complication of UGIB. UGIB can result in different clinical features of circulatory impairment, including abnormal hypertension or hypotension, decline or rise of heart and pulse rate, hypovolemic shock, and sudden cardiac arrest. According to a previous study, there are two distinct phases of the neurohumoral and hemodynamic responses in acute hemorrhage[38]: The initial arterial baroreceptor-mediated phase and the abruptly progressive phase characterized by withdrawal of sympathetic vasoconstrictor drive. In the initial phase, the medulla oblongata accepts the signals from carotid and aortic pressure receptors as well as from arterial che
Rebleeding has been indicated as a predictor for high mortality risk in patients with UGIB[45,46]. Based on the Forrest classification, endoscopic manifestations (i.e., active arterial bleeding, visible vessels, and adherent thrombosis) signify high rebleeding risk, with a rebleeding rate of 90%, 50%, and 33% in the absence of endoscopic hemostasis, respectively; these were much higher than those with flat pigmented spots and clean ulcer bases[47,48]. In our observation, the overall rebleeding rate of UGIB was 6.9%, with a significant elevation in the PP than in the GP group (3.2%: 20.9%; P < 0.001). Additionally, rebleeding was associated with the compound outcomes by Logistic univariate analysis. Rebleeding has been involved in most of the scoring systems; thus, we ultimately integrated rebleeding into our model for UGIB prognostication.
In recent years, the widespread use of endoscopy facilitates the microscopic diagnosis and hemostatic treatment of UGIB. A microscopic feature, SRH, remains of great importance and is recognized as an independent predictor for UGIB mortality. Moreover, it provides useful and important prognostic information for risk stratification and clinical decision making (e.g., receiving endoscopic hemostasis or surgical intervention or intervention)[14,49,50]. As expected, SRH was one of the most powerful predictors of poor UGIB prognosis in our model. Therefore, this finding encouraged early en
There are existing models for UGIB prognostication, including GBS, ABC, PNED, AIMS65, Rockall scores, and so on. Among these, the GBS score was initially established to predict the need for blood transfusion or intervention and to help outpatient emergency physicians to identifying those who need hospitalization. The ABC, PNED, AIMS65, and Rockall scores were established with death as the predictive endpoint event. A previous study showed that the predictive power of the AIMS65 and PNED scores were similar for 30-day mortality, which were better than the Rockall and GBS scores[18]. In our study, we formulated newer post-endoscopic (MH-STRALP) and pre-endoscopic (pre-MH-STRALP) scoring systems to predict the prognosis of UGIB by using compound endpoint events (i.e., undergoing emergency surgery or vascular intervention, being transferred to the ICU, or dying during hospitalization); we presented the scoring system as a nomogram. The AUC of the novel models were significantly higher than the other scores in both the training and va
There are some limitations in our study. Firstly, this is a retrospective study, which might lead to selection bias. Se
Taken together, we successfully developed novel post-endoscopic (MH-STRALP) and pre-endoscopic (pre-MH-STRALP) scoring systems to conveniently and accurately predict the prognosis of UGIB. Further large-scale prospective studies are necessary to verify the value of the scor
Upper gastrointestinal bleeding (UGIB) is divided into nonvariceal UGIB (NVUGIB) and variceal UGIB (VUGIB), with rising NVUGIB cases due to an aging population and more non-steroidal anti-inflammatory drugs use, and VUGIB typically resulting from liver cirrhosis with substantial mortality. Despite medical and endoscopic progress, UGIB main
UGIB prognostic scoring systems are inconsistently applied in clinical practice, with limited impact on decision-making due to variances in primary outcomes and lack of validation. The absence of trial-based evidence for these scores con
The goal of this study is to identify key factors that influence poor UGIB outcomes (e.g., the demand for emergency sur
A retrospective study used UGIB patient data from East Hospital as a training cohort and from Changzheng Hospital as a validation cohort to construct and test a new scoring model based on major predictors of UGIB outcomes, including en
Univariate analysis determined factors related to negative outcomes in UGIB, leading to the creation of the MH-STRALP scoring system, which incorporates seven prognostically significant factors plus the risk of rebleeding. The MH-STRALP system showed better prognostic accuracy compared to other established scoring systems (GBS, Rockall, ABC, AIMS65, and PNED), with areas under ROC curves (AUROC) of 0.899 and 0.826 in the training and validation cohorts, whilst the pre-MH-STRALP score also showed better predictive value (AUROCs of 0.868 and 0.767 in the training and validation cohorts, respectively).These two scoring systems are helpful in prognosticating Chinese UGIB patients, providing personalized appropriate treatment and management, and facilitating early clinical decision-making.
With this study, we constructed new scoring systems, MH-STRALP and pre-MH-STRALP, to predict the prognosis of patients with UGIB, with compound endpoint events of undergoing emergency surgery or vascular intervention, being transferred to the ICU, or dying during hospitalization; we formulated a nomogram to present the scoring system.
Since this is a retrospective study and we did not make prognostic predictions for patients with UGIB who did not un
The authors would like to thank the patients and their families for their contribution to this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Christodoulidis G, Greece S-Editor: Lin C L-Editor: A P-Editor: Xu ZH
| 1. | Kamboj AK, Hoversten P, Leggett CL. Upper Gastrointestinal Bleeding: Etiologies and Management. Mayo Clin Proc. 2019;94:697-703. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Hreinsson JP, Kalaitzakis E, Gudmundsson S, Björnsson ES. Upper gastrointestinal bleeding: incidence, etiology and outcomes in a population-based setting. Scand J Gastroenterol. 2013;48:439-447. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Rotondano G. Epidemiology and diagnosis of acute nonvariceal upper gastrointestinal bleeding. Gastroenterol Clin North Am. 2014;43:643-663. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Herrera JL. Management of acute variceal bleeding. Clin Liver Dis. 2014;18:347-357. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789-1858. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Tielleman T, Bujanda D, Cryer B. Epidemiology and Risk Factors for Upper Gastrointestinal Bleeding. Gastrointest Endosc Clin N Am. 2015;25:415-428. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Wuerth BA, Rockey DC. Changing Epidemiology of Upper Gastrointestinal Hemorrhage in the Last Decade: A Nationwide Analysis. Dig Dis Sci. 2018;63:1286-1293. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Jairath V, Rehal S, Logan R, Kahan B, Hearnshaw S, Stanworth S, Travis S, Murphy M, Palmer K, Burroughs A. Acute variceal haemorrhage in the United Kingdom: patient characteristics, management and outcomes in a nationwide audit. Dig Liver Dis. 2014;46:419-426. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Sung JJ, Chiu PW, Chan FKL, Lau JY, Goh KL, Ho LH, Jung HY, Sollano JD, Gotoda T, Reddy N, Singh R, Sugano K, Wu KC, Wu CY, Bjorkman DJ, Jensen DM, Kuipers EJ, Lanas A. Asia-Pacific working group consensus on non-variceal upper gastrointestinal bleeding: an update 2018. Gut. 2018;67:1757-1768. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, Rotondano G, Hucl T, Dinis-Ribeiro M, Marmo R, Racz I, Arezzo A, Hoffmann RT, Lesur G, de Franchis R, Aabakken L, Veitch A, Radaelli F, Salgueiro P, Cardoso R, Maia L, Zullo A, Cipolletta L, Hassan C. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345-60; quiz 361. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Laine L, Barkun AN, Saltzman JR, Martel M, Leontiadis GI. ACG Clinical Guideline: Upper Gastrointestinal and Ulcer Bleeding. Am J Gastroenterol. 2021;116:899-917. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Marmo R, Koch M, Cipolletta L, Capurso L, Grossi E, Cestari R, Bianco MA, Pandolfo N, Dezi A, Casetti T, Lorenzini I, Germani U, Imperiali G, Stroppa I, Barberani F, Boschetto S, Gigliozzi A, Gatto G, Peri V, Buzzi A, Della Casa D, Di Cicco M, Proietti M, Aragona G, Giangregorio F, Allegretta L, Tronci S, Michetti P, Romagnoli P, Piubello W, Ferri B, Fornari F, Del Piano M, Pagliarulo M, Di Mitri R, Trallori G, Bagnoli S, Frosini G, Macchiarelli R, Sorrentini I, Pietrini L, De Stefano S, Ceglia T, Chiozzini G, Salvagnini M, Di Muzio D, Rotondano G; Italian registry on upper gastrointestinal bleeding (Progetto Nazionale Emorragie Digestive--PNED 2). Predicting mortality in non-variceal upper gastrointestinal bleeders: validation of the Italian PNED Score and Prospective Comparison with the Rockall Score. Am J Gastroenterol. 2010;105:1284-1291. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316-321. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356:1318-1321. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Saltzman JR, Tabak YP, Hyett BH, Sun X, Travis AC, Johannes RS. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc. 2011;74:1215-1224. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Laursen SB, Oakland K, Laine L, Bieber V, Marmo R, Redondo-Cerezo E, Dalton HR, Ngu J, Schultz M, Soncini M, Gralnek I, Jairath V, Murray IA, Stanley AJ. ABC score: a new risk score that accurately predicts mortality in acute upper and lower gastrointestinal bleeding: an international multicentre study. Gut. 2021;70:707-716. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Stanley AJ, Laine L, Dalton HR, Ngu JH, Schultz M, Abazi R, Zakko L, Thornton S, Wilkinson K, Khor CJ, Murray IA, Laursen SB; International Gastrointestinal Bleeding Consortium. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: international multicentre prospective study. BMJ. 2017;356:i6432. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Li Y, Lu Q, Song M, Wu K, Ou X. Comparisons of six endoscopy independent scoring systems for the prediction of clinical outcomes for elderly and younger patients with upper gastrointestinal bleeding. BMC Gastroenterol. 2022;22:187. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Stanley AJ, Dalton HR, Blatchford O, Ashley D, Mowat C, Cahill A, Gaya DR, Thompson E, Warshow U, Hare N, Groome M, Benson G, Murray W. Multicentre comparison of the Glasgow Blatchford and Rockall Scores in the prediction of clinical end-points after upper gastrointestinal haemorrhage. Aliment Pharmacol Ther. 2011;34:470-475. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Iino C, Mikami T, Igarashi T, Aihara T, Ishii K, Sakamoto J, Tono H, Fukuda S. Evaluation of scoring models for identifying the need for therapeutic intervention of upper gastrointestinal bleeding: A new prediction score model for Japanese patients. Dig Endosc. 2016;28:714-721. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Nahm FS. Receiver operating characteristic curve: overview and practical use for clinicians. Korean J Anesthesiol. 2022;75:25-36. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA; American College of Gastroenterology. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679-92; quiz 693. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Kim JS, Kim BW, Kim DH, Park CH, Lee H, Joo MK, Jung DH, Chung JW, Choi HS, Baik GH, Lee JH, Song KY, Hur S. Guidelines for Nonvariceal Upper Gastrointestinal Bleeding. Gut Liver. 2020;14:560-570. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Kim YI, Choi IJ, Cho SJ, Lee JY, Kim CG, Kim MJ, Ryu KW, Kim YW, Park YI. Outcome of endoscopic therapy for cancer bleeding in patients with unresectable gastric cancer. J Gastroenterol Hepatol. 2013;28:1489-1495. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Sheibani S, Kim JJ, Chen B, Park S, Saberi B, Keyashian K, Buxbaum J, Laine L. Natural history of acute upper GI bleeding due to tumours: short-term success and long-term recurrence with or without endoscopic therapy. Aliment Pharmacol Ther. 2013;38:144-150. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Kaviani MJ, Pirastehfar M, Azari A, Saberifiroozi M. Etiology and outcome of patients with upper gastrointestinal bleeding: a study from South of Iran. Saudi J Gastroenterol. 2010;16:253-259. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Roberts SE, Button LA, Williams JG. Prognosis following upper gastrointestinal bleeding. PLoS One. 2012;7:e49507. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Walayat S, Martin D, Patel J, Ahmed U, N Asghar M, Pai AU, Dhillon S. Role of albumin in cirrhosis: from a hospitalist's perspective. J Community Hosp Intern Med Perspect. 2017;7:8-14. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Bernardi M, Angeli P, Claria J, Moreau R, Gines P, Jalan R, Caraceni P, Fernandez J, Gerbes AL, O'Brien AJ, Trebicka J, Thevenot T, Arroyo V. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut. 2020;69:1127-1138. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Fernández J, Clària J, Amorós A, Aguilar F, Castro M, Casulleras M, Acevedo J, Duran-Güell M, Nuñez L, Costa M, Torres M, Horrillo R, Ruiz-Del-Árbol L, Villanueva C, Prado V, Arteaga M, Trebicka J, Angeli P, Merli M, Alessandria C, Aagaard NK, Soriano G, Durand F, Gerbes A, Gustot T, Welzel TM, Salerno F, Bañares R, Vargas V, Albillos A, Silva A, Morales-Ruiz M, Carlos García-Pagán J, Pavesi M, Jalan R, Bernardi M, Moreau R, Páez A, Arroyo V. Effects of Albumin Treatment on Systemic and Portal Hemodynamics and Systemic Inflammation in Patients With Decompensated Cirrhosis. Gastroenterology. 2019;157:149-162. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58:1836-1846. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Dong J, Xu XH, Ke MY, Xiang JX, Liu WY, Liu XM, Wang B, Zhang XF, Lv Y. The FIB-4 score predicts postoperative short-term outcomes of hepatocellular carcinoma fulfilling the milan criteria. Eur J Surg Oncol. 2016;42:722-727. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Meyer JS, Stoica E, Pascu I, Shimazu K, Hartmann A. Catecholamine concentrations in CSF and plasma of patients with cerebral infarction and haemorrhage. Brain. 1973;96:277-288. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Lewis EA. Gastroduodenal ulceration and haemorrhage of neurogenic origin. Br J Surg. 1973;60:279-283. [PubMed] [Cited in This Article: ] |
| 36. | Schaller BJ, Graf R, Jacobs AH. Pathophysiological changes of the gastrointestinal tract in ischemic stroke. Am J Gastroenterol. 2006;101:1655-1665. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Chen CM, Hsu HC, Chuang YW, Chang CH, Lin CH, Hong CZ. Study on factors affecting the occurrence of upper gastrointestinal bleeding in elderly acute stroke patients undergoing rehabilitation. J Nutr Health Aging. 2011;15:632-636. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Schadt JC, Ludbrook J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am J Physiol. 1991;260:H305-H318. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Sander-Jensen K, Secher NH, Astrup A, Christensen NJ, Giese J, Schwartz TW, Warberg J, Bie P. Hypotension induced by passive head-up tilt: endocrine and circulatory mechanisms. Am J Physiol. 1986;251:R742-R748. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev. 1990;70:963-985. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | LANDGREN S, NEIL E. Chemoreceptor impulse activity following haemorrhage. Acta Physiol Scand. 1951;23:158-167. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | Restellini S, Kherad O, Jairath V, Martel M, Barkun AN. Red blood cell transfusion is associated with increased rebleeding in patients with nonvariceal upper gastrointestinal bleeding. Aliment Pharmacol Ther. 2013;37:316-322. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Chen YC, Hsiao CT, Lin LC, Hsiao KY, Hung MS. The association between red blood cell transfusion and outcomes in patients with upper gastrointestinal bleeding. Clin Transl Gastroenterol. 2018;9:138. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Hearnshaw SA, Logan RF, Palmer KR, Card TR, Travis SP, Murphy MF. Outcomes following early red blood cell transfusion in acute upper gastrointestinal bleeding. Aliment Pharmacol Ther. 2010;32:215-224. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Ardevol A, Alvarado-Tapias E, Garcia-Guix M, Brujats A, Gonzalez L, Hernández-Gea V, Aracil C, Pavel O, Cuyas B, Graupera I, Colomo A, Poca M, Torras X, Concepción M, Villanueva C. Early rebleeding increases mortality of variecal bleeders on secondary prophylaxis with β-blockers and ligation. Dig Liver Dis. 2020;52:1017-1025. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | El Hajj W, Quentin V, Boudoux D'Hautefeuille G, Vandamme H, Berger C, Moussaoui MR, Berete A, Louvel D, Bertolino JG, Cuillerier E, Thiebault Q, Arondel Y, Grimbert S, Le Guillou B, Borel I, Lahmek P, Nahon S; ANGH for the SANGHRIA Study Group. Prognosis of variceal and non-variceal upper gastrointestinal bleeding in already hospitalised patients: Results from a French prospective cohort. United European Gastroenterol J. 2021;9:707-717. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Jensen DM, Machicado GA. Endoscopic Hemostasis of Ulcer Hemorrhage with Injection, Thermal, and Combination Methods. Tech Gastrointest Endosc. 2005;7:124-131. [DOI] [Cited in This Article: ] |
| 48. | Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2:394-397. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Kovacs TO, Jensen DM. Endoscopic treatment of ulcer bleeding. Curr Treat Options Gastroenterol. 2007;10:143-148. [PubMed] [DOI] [Cited in This Article: ] |
| 50. | Freeman ML. Stigmata of hemorrhage in bleeding ulcers. Gastrointest Endosc Clin N Am. 1997;7:559-574. [PubMed] [Cited in This Article: ] |