Published online Feb 27, 2024. doi: 10.4240/wjgs.v16.i2.382
Peer-review started: November 7, 2023
First decision: December 17, 2023
Revised: December 25, 2023
Accepted: February 1, 2024
Article in press: February 1, 2024
Published online: February 27, 2024
The systemic inflammatory response index (SIRI) has been demonstrated to make a significant difference in assessing the prognosis of patients with different solid neoplasms. However, research is needed to ascertain the accuracy and reliability of applying the SIRI to patients who undergo robotic radical gastric cancer sur
To validate the applicability of the SIRI in assessing the survival of gastric cancer patients and evaluate the clinical contribution of preoperative SIRI levels to predicting long-term tumor outcomes in patients, who received robotic radical gastric cancer surgery.
Initially, an exhaustive retrieval was performed in the PubMed, the Cochrane Library, EMBASE, Web of Science, and Scopus databases to identify relevant studies. Subsequently, a meta-analysis was executed on 6 cohort studies iden
The findings demonstrated an extensive connection between SIRI values and the outcome of patients with gastric cancer. Preoperative SIRI levels were identified as an independent hazard feature for both OS and DFS among those who received robotic surgery for gastric cancer. SIRI levels in gastric cancer patients were observed to be associated with the presence of comorbidities, T-stage, carcinoembryonic antigen levels, the development of early serious postoperative complications, and the rate of lymph node metastasis.
SIRI values are correlated with adverse in the gastric cancer population and have the potential to be utilized in predicting long-term oncological survival in patients who undergo robotic radical gastric cancer surgery.
Core Tip: The aim of this study was to assess the clinical importance and prognostic significance of systemic inflammatory response index (SIRI) on postoperative outcomes in patients with robotic gastrectomy. We collected all the available data in a meta-analysis, and then retrospectively collected baseline data to further explore the relationship of SIRI values with clinicopathological characteristics and prognosis. The results discovered that SIRI values were an independent hazard feature for both overall survival and disease-free survival among those who received robotic surgery. Evaluation of SIRI value levels can help surgeons and oncologists to more effectively assess preoperative treatment and develop postoperative management strategies for gastric cancer patients.
- Citation: Ren JY, Xu M, Niu XD, Ma SX, Jiao YJ, Wang D, Yu M, Cai H. Systemic inflammatory response index is a predictor of prognosis in gastric cancer patients: Retrospective cohort and meta-analysis. World J Gastrointest Surg 2024; 16(2): 382-395
- URL: https://www.wjgnet.com/1948-9366/full/v16/i2/382.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i2.382
Gastric cancer, driven by high disease burden and lethality, pose an urgent issue for the community[1]. It is considered the third major cause of mortality and the fifth most prevalent malignancy among oncology patients[2]. Tremendous surgical advancements in the management of gastric cancer have been made across the last few decades, with the emergence of robotic gastric surgery as an undeniably powerful advantage[3]. Findings indicate that robotic gastrectomy may be of additional worth in lymph node dissection and early postoperative course[4]. Although the effectiveness of enhanced chemotherapy and surgical alternatives has been demonstrated in these patients, the prognosis is still unsatisfactory[1]. The Correa sequence, a classical model of gastric cancer progression, states that the inflammatory response is a crucial contributor to the tumor advancement process. Chronic inflammation of the gastric mucosa can contribute to the development of gastric cancer[5], by releasing systemic biochemical cytokines in response to inflammation, and the resulting carcinoma cell development drives postoperative recurrence and metastasis[6]. A growing body of recent research revealed that tumor-associated inflammatory fluences were strongly relevant to the survival and probability in cancer patients[7]. The systemic inflammatory response index (SIRI) is emerging as a comprehensive parameter which is calculated by neutrophil count × monocyte count/Lymphocyte count, and it is capable of recognizing inflammatory conditions in individuals. The SIRI has also shown considerable value in the prognostic assessment of patients with many solid tumors, such as lung cancer[8], liver cancer[9], tongue cancer[10], and bladder cancer[11]. Therefore, to more accurately understand the prognostic relevance of peripheral blood SIRI values in gastric cancer patients, we collected all the available data in a meta-analysis and then retrospectively collected baseline data from patients who underwent robotic radical gastric surgery to further explore the relationship between SIRI values, clinicopathological characteristics, and patient prognosis.
Inclusion and exclusion criteria: The inclusive criteria were: (1) A connection between serum SIRI values and the overall survival (OS) or disease-free survival (DFS) of patients with gastric cancer was reported; (2) an assessment of the hazard ratios (HR) and the corresponding 95% confidence intervals (CI) was available; and (3) the full text was accessible. The exclusion criteria were: (1) Unoriginal articles (reviews, commentaries, editorials, conference abstracts, and case reports; (2) articles without sufficient HR survival data; and (3) duplicate studies.
Search procedure and study selection: The process of searching and selecting the studies for this meta-analysis adhered to the guidelines of the Preferred Reporting Items for Systematic Evaluation and Meta-Analyses. The main search terms used were "gastric cancer", "stomach neoplasm" and "systemic inflammatory response index". Detailed search strategies can be found in the Supplementary material.
Data extraction: Two investigators independently collected two aspects of data from the included studies: (1) Fun
Data analysis: A meta-analysis was conducted using Stata statistical software to examine the included studies. The I2 and P coefficient were utilized to evaluate the heterogeneity among the studies. An the I2 value equal to or below 50% and a P value equal to or above 0.1, was deemed acceptable for implementing the fixed effect model (FEM). Conversely, if the heterogeneity was determined to be substantial, the random effect model was employed. Numerical data are described by relative risk, odds ratio, and 95%CI. A P value of < 0.05 was designated as statistical significance.
Data collection: We conducted an investigation of patients with histologically verified gastric cancer from January 2018 to December 2019 in Gansu Provincial People's Hospital. The investigation followed the principles of the Declaration of Helsinki and obtained approval was obtained from the Ethics Committee The study was conducted according to the approved protocol (ethical consent: 21/10/2022-410, Medical Ethics Committee of Gansu Provincial Hospital). Data on gender, age, tumor diameter, tumor location, lymph node metastasis rate, degree of differentiation, immunohistochemical results (ki67, P53, and Her2), tumor-node-metastasis (TNM) staging (with reference to the American Joint Committee on Cancer (AJCC) criteria for TNM gastric cancer staging (8th ed.)], American society of anesthesiology scores, surgical approach, extent of the resection, duration of surgery, blood loss, and perioperative blood transfusion, length of hospital stay, duration of postoperative enteral nutrition and early postoperative complications were gathered from the electronic medical database. The longest diameter measured in routine postoperative pathological specimens was used as the tumor size. The primary tumor location was classified as upper, middle and hypogastric. The degree of differentiation was classified as low/moderate differentiation and high differentiation. Comorbidities were defined in patients with one or more conditions that included of hypertension, diabetes mellitus, and coronary heart disease. Early postoperative complications were defined as those that developed while hospitalized or within 30 d of having surgery, and all complications were graded in terms of severity based on the Clavien-Dindo classification. Grade I or II complications were classified as mild and grade III and higher complications considered severe. Blood specimens were collected from patients within a week prior to surgery to obtain laboratory parameters values.
The criteria for inclusion were: (1) Between 18 and 80 years of age with a clear preoperative clinical diagnosis and post
All statistical calculations were performed using IBM SPSS Statistics for Windows version 26.0 (IBM Corporation, Armonk, NY, United States). Categorical information is presented as the number of cases (n) and percentages (%). For quantitative data, non-normally distributed continuous-type information is expressed by the medians and interquartile ranges, and normally distributed measures are described as the mean ± SD. Either the Student's t-test or Mann-Whitney U test was used to compare groups, depending on the normality of the statistic distribution. The χ2 test was utilized to detect disparities among the classification groups. The optimal threshold for each outcome was determined using the receiver operating characteristic (ROC) curve with the Youden index. Cox proportional hazards models were utilized to estimate the risk ratio for OS and DFS, with HR and 95%CIs reported. A statistically significant level was defined as P < 0.05.
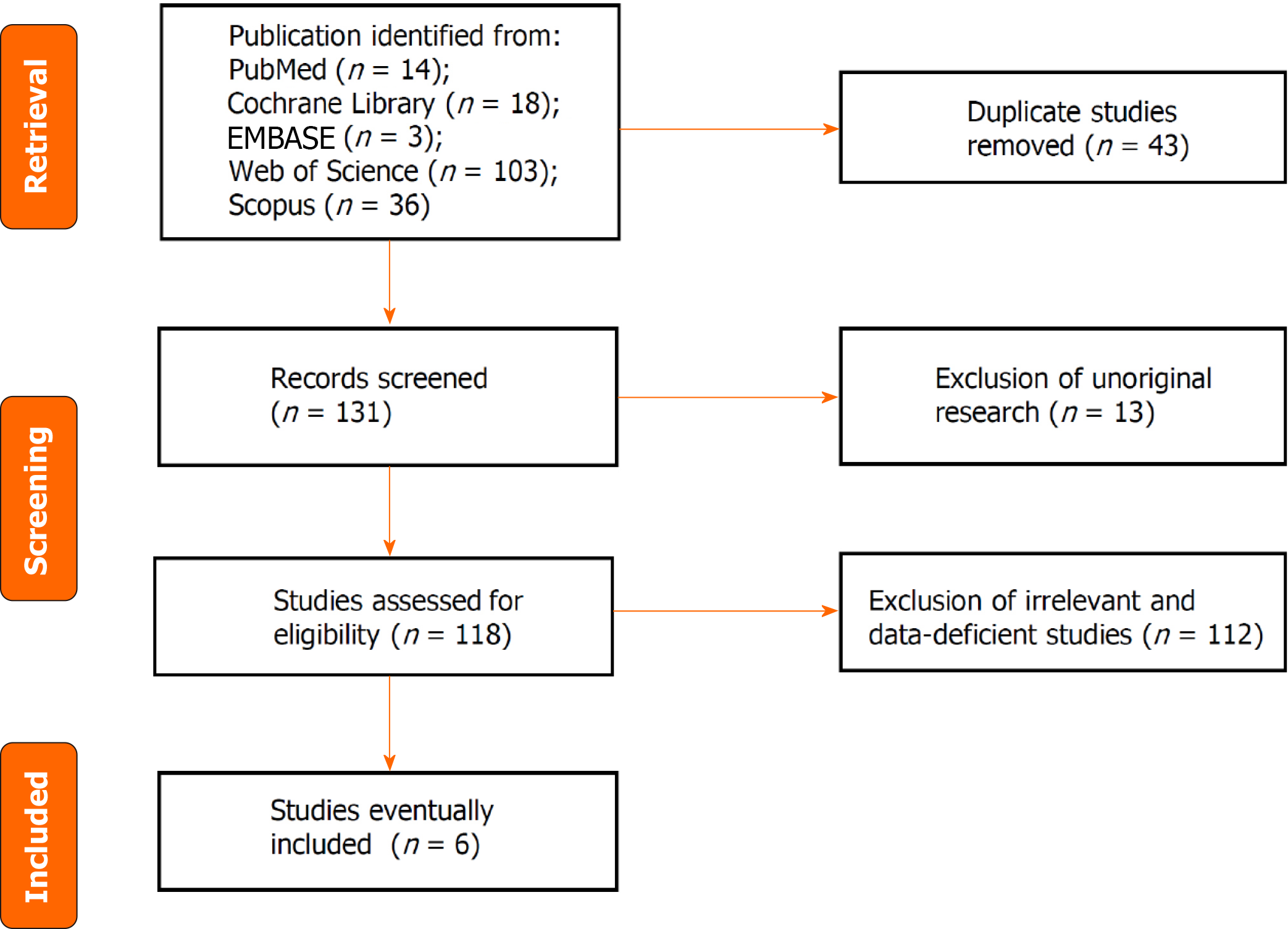
Literature search results: A total of 174 articles were identified by the search strategy, including 14 articles in PubMed, 18 articles in the Cochrane Library, 3 articles in EMBASE, 103 articles in the Web of Science and 36 articles in Scopus. Forty-three duplicate articles were deleted, 111 were excluded by evaluating the title and abstract, and 14 were excluded by reading the full text, of which only 6 articles contained risk ratio information and were eligible for the survival outcome analysis. There were 2114 cases overall. The process of literature screening is illustrated in Figure 1. Table 1 presents fundamental descriptions of the included studies.
| Population | Patients | Included years | Detection method | Cut-off value | Survival type (analysis type) | Treatments | Follow- up | HR | LL | UL | NOS score |
| Localized or regional gastric cancer | 782 | 2007-2009/2010-2011 | ROC analysis | 0.82 | DFS/DSS | With surgery | ≥ 5 yr | DFS 2.529 | DFS 1.922 | DFS 3.326 | 7 |
| Gastric cancer | 594 | 2011-2016 | ROC analysis | 0.85 | OS | With surgery | ≥ 5 yr | OS 2.06 | OS 1.6 | OS 2.66 | 8 |
| Respectable gastric cancer | 240 | 2007-2016 | X-tile | 1.2 | DFS/OS | With surgery | ≥ 5 yr | OS 1.999/DFS 1.964 | OS 1.387/DFS 1.363 | OS 2.881/DFS 2.828 | 8 |
| Locally advanced gastric cancer | 89 | 2010-2018 | ROC analysis | 0.58 | DFS | With surgery and adjuvant chemoradiotherapy | < 5 yr | DFS 3.002 | DFS 0.912 | DFS 9.882 | 7 |
| Received neoadjuvant chemotherapy gastric cancer | 107 | 2007-2015 | ROC analysis | 1.21 | DFS/OS | With neoadjuvant chemotherapy and surgery | ≥ 5 yr | OS 3.331/DFS 3.437 | OS 1.001/DFS 1.059 | OS 11.082/DFS 11.149 | 8 |
| Adenocarcinoma of the esophagogastric junction | 302 | 2008-2010 | ROC analysis | 0.68 | OS | With neoadjuvant chemotherapy and surgery | ≥ 5 yr | OS 1.9 | OS 1.36 | OS 2.64 | 8 |
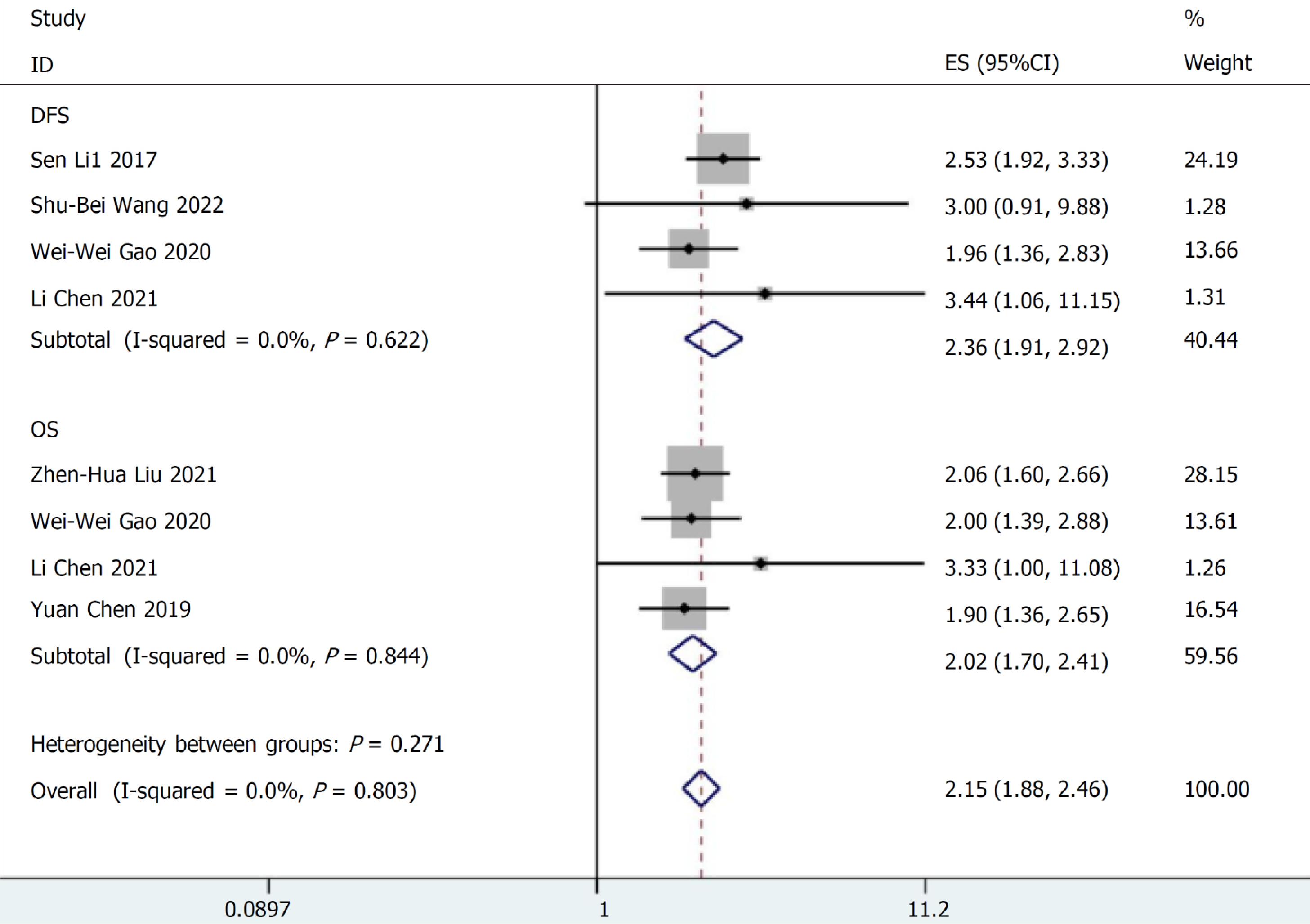
SIRI and prognosis of gastric cancer patients: Two studies involving 347 cases described the relationship between SIRI values and OS and DFS. They were combined for meta-analysis. No discernible variability was found between the studies existed (I2 < 0.001%, P = 0.271), and the FEM was applied to merge the HR and 95%CI. The findings of the meta-analysis demonstrated that a higher SIRI value was consistently correlated with a poor survival in cancer patients (DFS: HR = 2.36, 95%CI: 1.91-2.92; P < 0.001; OS: HR = 2.02, 95%CI: 1.70-2.41; P < 0.001, Figure 2).
Baseline materials: A total of 161 participants were included in the retrospective analysis. Table 2 displays the baseline data and clinicopathological traits of the included patients. The study involved 128 males and 33 females. The average age of the subjects was 61.80 ± 9.26 years (30-84 years). The mean preoperative body mass index of all patients was 22.30 ± 3.35 kg/m2. In accordance with the AJCC staging criteria 8th edition, stages I, II, and III were assigned to 26 (16.1%), 49 (30.4%), and 86 (53.4%) patients, respectively. The average follow-up interval for all patients was 42 months (6-60 months). The ideal SIRI cut-off value in ROC analysis was 1.38. By applying the cutoff, 106 (65.8%) individuals had high SIRI values, and 55 (34.2%) with low SIRI values. Preoperative SIRI levels in gastric cancer patients were linked to the presence of comorbidities (P = 0.006), T-stage (P = 0.015), carcinoembryonic antigen (CEA) level (P = 0.003), the presence of early serious postoperative complications (P = 0.001), and the lymph node invasion rate (P = 0.023) (Table 2).
| Variables | SIRI ≤ 1.38 (n = 106) | SIRI > 1.38 (n = 55) | P value | |
| Gender | 0.0791 | |||
| Male | 80 | 48 | ||
| Female | 26 | 7 | ||
| Age (yr) | 60.80 (12) | 63.71 (14) | 0.0582 | |
| Underlying disease | No | 91 | 37 | 0.0061 |
| Yes | 15 | 18 | ||
| BMI | 22.36 (4.7) | 22.19 (4.2) | 0.7652 | |
| Tumor location | Upper third | 12 | 7 | 0.9381 |
| Middle third | 9 | 4 | ||
| Lower third | 85 | 44 | ||
| Tumor dimensions (cm) | 0.0561 | |||
| < 3 | 32 | 9 | ||
| ≥ 3 | 74 | 46 | ||
| Differentiation | 0.1661 | |||
| Moderate and poor | 101 | 55 | ||
| Well | 5 | 0 | ||
| T stage | 0.0151 | |||
| T1 | 17 | 4 | ||
| T2 | 11 | 0 | ||
| T3 | 53 | 31 | ||
| T4 | 25 | 20 | ||
| N stage | 0.3381 | |||
| N0 | 40 | 13 | ||
| N1 | 19 | 11 | ||
| N2 | 13 | 9 | ||
| N3 | 34 | 22 | ||
| TNM stage | 0.0861 | |||
| I | 22 | 4 | ||
| II | 31 | 18 | ||
| III | 53 | 33 | ||
| Operation | 0.4471 | |||
| Subtotal gastrectomy | 51 | 23 | ||
| Total gastrectomy | 55 | 32 | ||
| Operation time (minutes) | ||||
| ASA | 0.7961 | |||
| I-II | 95 | 50 | ||
| III-IV | 11 | 5 | ||
| Perioperative transfusion | 0.141 | |||
| No | 86 | 39 | ||
| Yes | 20 | 16 | ||
| CA199 (ng/mL) | 0.0771 | |||
| Negative | 91 | 41 | ||
| Positive | 15 | 14 | ||
| CEA (ng/mL) | 0.0031 | |||
| Negative | 88 | 34 | ||
| Positive | 18 | 21 | ||
| Blood loss (mL) | 0.0531 | |||
| < 200 | 79 | 31 | ||
| 200 ≤ X ≤ 400 | 24 | 20 | ||
| > 400 | 3 | 4 | ||
| Postoperative complication | 0.0011 | |||
| No | 99 | 41 | ||
| Yes | 7 | 14 | ||
| Postoperative chemotherapy | 0.9251 | |||
| No | 32 | 17 | ||
| Yes | 74 | 38 | ||
| P53 | 0.7621 | |||
| Wild | 36 | 20 | ||
| Mutant | 70 | 35 | ||
| Ki-67 | 0.7721 | |||
| 0%-49% | 7 | 5 | ||
| 50%-74% | 34 | 19 | ||
| 75%-100% | 65 | 31 | ||
| Her-2 | 0.7741 | |||
| Negative | 99 | 52 | ||
| Positive | 7 | 3 | ||
| Lymph node metastasis rate (%) | 0.0231 | |||
| 0.00% | 42 | 12 | ||
| > 0.00% | 64 | 43 | ||
| Enteral nutrition time | 0.3181 | |||
| ≤ 7 d | 73 | 42 | ||
| > 7 d | 33 | 13 | ||
| Duration of surgery (minutes) | 267.3 (90) | 269.7 (60) | 0.8012 | |
| Length of hospitalization (days) | 17.89 (5) | 18.04 (6) | 0.8762 | |
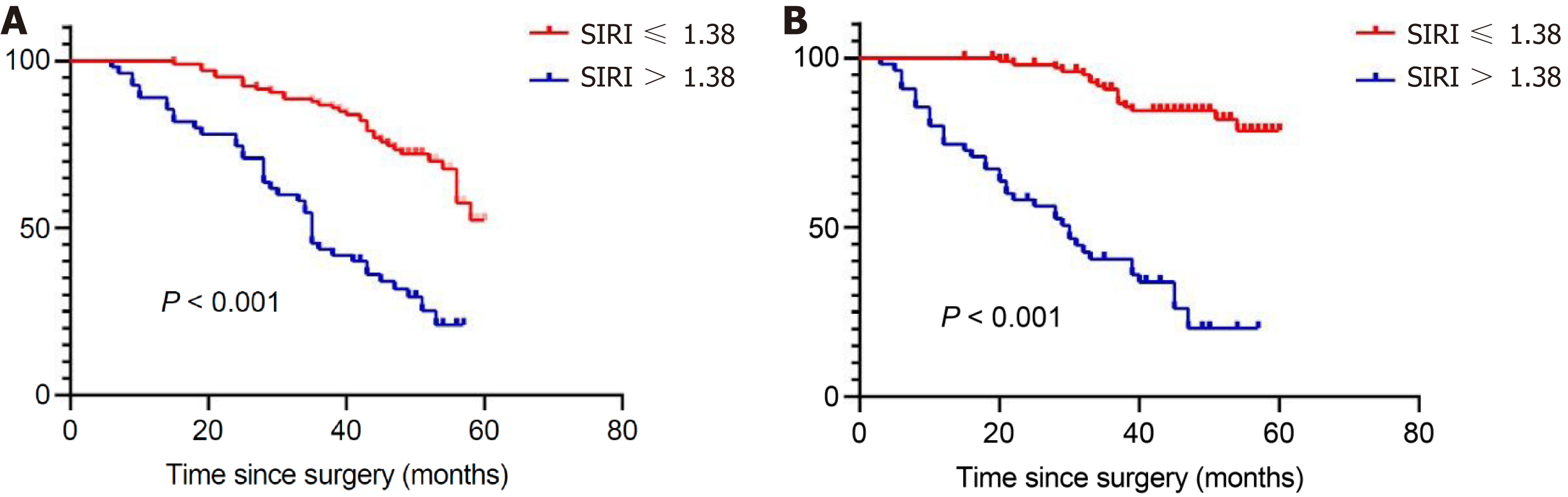
Cox regression survival analysis: Among the included gastric cancer patients, univariate analysis revealed that older age (P < 0.001), large tumor volume (P = 0.025), late clinical stage (P = 0.005), positive CEA (P < 0.001), intrasurgical blood loss > 200 mL (P = 0.016), lymph node metastasis greater than 4.6% (P < 0.001), serious complications after surgery (P < 0.001), and no complementary chemotherapy (P = 0.001) were associated with shorter 5-year postoperative survival time. The multifactorial model demonstrated that age (HR: 1.067, 95%CI: 1.035-1.099; P < 0.001), TNM stage (P = 0.025), the occurrence of serious complications after surgery (HR: 7.244, 95%CI: 3.780-13.781; P < 0.001), P53 mutation (HR: 0.392, 95%CI: 0.231-0.663; P < 0.001), and Ki67 levels (P = 0.003) were influential prognostic variables for 5-year OS (Table 3). Similarly, COX survival analysis indicated that age (P = 0.003), the presence of comorbidities (P = 0.045), TNM stage (P = 0.003), and the presence of serious postoperative complications (P < 0.001), were individual prognostic factors for DFS (Supplementary Table 1). In univariate analysis, lower SIRI values were associated with longer OS and DFS (HR: 3.787, 95%CI: 2.376-6.034, P < 0.001; HR: 8.884, 95%CI: 4.735-15.070, P < 0.001). According to the multivariable analysis, patients in the low SIRI group had improved survival results. (HR: 2.696, 95%CI: 1.579-4.404, P < 0.001; HR: 6.406, 95%CI: 3.370-12.178, P < 0.001). Further, The OS and DFS Kaplan-Meier survival curves for the SIRI values of all patients are illustrated in Figure 3.
| Variables | Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI value | P value | HR | 95%CI | P value | ||
| Gender | Male/female | 0.743 | 0.400-1.379 | 0.346 | |||
| Age (yr) | 1.05 | 1.023-1.077 | < 0.001 | 1.067 | 1.035-1.099 | < 0.001 | |
| Underlying disease | No/yes | 1.307 | 0.759-2.248 | 0.334 | |||
| BMI | 0.98 | 0.915-1.048 | 0.553 | ||||
| Tumor location | 0.264 | ||||||
| Upper third | Ref | ||||||
| Middle third | 0.434 | 0.117-1.604 | 0.211 | ||||
| Lower third | 1.13 | 0.379-2.274 | 0.733 | ||||
| Tumor dimensions (cm) | < 3/≥ 3 | 0.494 | 0.266-0.917 | 0.025 | 0.672 | 0.338-1.338 | 0.258 |
| Differentiation | Moderate and poor/well | 0.047 | 0.000-11.342 | 0.275 | |||
| TNM stage | 0.005 | 0.025 | |||||
| I | Ref | Ref | |||||
| II | 3.77 | 1.264-8.709 | 0.042 | 3.03 | 1.243-7.984 | 0.047 | |
| III | 5.695 | 2.181-9.845 | 0.001 | 5.68 | 2.161-9.541 | 0.01 | |
| Operation | Subtotal gastrectomy /total gastrectomy | 1.423 | 0.893-2.269 | 0.138 | |||
| ASA | I-II/III-IV | 1.118 | 0.535-2.336 | 0.767 | |||
| Perioperative transfusion | No/yes | 1.58 | 0.959-2.603 | 0.073 | |||
| Blood loss (mL) | > 200 mL/≤ 200 mL | 1.763 | 1.109-2.803 | 0.016 | 1.498 | 0.914-2.458 | 0.109 |
| Operation time (minutes) | 1.001 | 0.997-1.004 | 0.938 | ||||
| CA199 (ng/mL) | Negative/positive | 1.347 | 0.780-2.327 | 0.286 | |||
| CEA (ng/mL) | Negative/positive | 2.473 | 1.543-3.963 | < 0.001 | 1.266 | 0.76-2.108 | 0.365 |
| Postoperative complication | No/yes | 4.787 | 2.846-8.052 | < 0.001 | 7.244 | 3.780-13.781 | < 0.001 |
| Postoperative chemotherapy | No/yes | 2.929 | 1.543-5.562 | 0.001 | 1.298 | 0.649-2.597 | 0.461 |
| P53 | Wild/Mutant | 0.728 | 0.455-1.165 | 0.186 | 0.392 | 0.231-0.663 | < 0.001 |
| Ki-67 | 0.137 | 0.003 | |||||
| 0%-49% | Ref | Ref | |||||
| 50%-74% | 1.137 | 0.471-2.743 | 0.775 | 2.088 | 0.822-5.303 | 0.121 | |
| 75%-100% | 0.702 | 0.297-1.661 | 0.421 | 0.788 | 0.321-1.932 | 0.603 | |
| Her-2 | Negative/positive | 0.321 | 0.079-1.310 | 0.113 | |||
| Lymph node metastasis rate (%) | < 4.6%/≥ 4.6% | 5.943 | 2.845-12.414 | < 0.001 | 2.266 | 0.897-5.724 | 0.083 |
| Enteral nutrition time | 1.023 | 0.965-1.186 | 0.443 | ||||
| Length of hospitalization (days) | 1.032 | 0.995-1.070 | 0.092 | ||||
| SIRI | ≤ 1.38/> 1.38 | 3.787 | 2.376-6.034 | < 0.001 | 2.696 | 1.579-4.404 | < 0.001 |
According to the Cox regression model analysis, age, TNM stage, and severe postoperative complications were important factors affecting prognosis. Therefore, we performed further subgroup analyses. The participants were categorized into senior and junior age cohorts using 60 years as the cut-off age. The findings demonstrated that, in the junior and senior age categories, OS and DFS, respectively, were longer in the low SIRI value group (Figure 4A-D). In contrast, the individuals with SIRI value less than 1.38 exhibited longer OS and DFS in the TNM stage I and stage II subgroups (Figure 4E and F). In the TNM stage III group, patients with low levels of SIRI levels also had a relatively better prognosis (Figure 4G and H). Not surprisingly, OS and DFS were longer in the low SIRI group than in the high SIRI group compared to the subgroups with no or minor postoperative complications, respectively (Figure 4I and J), and with the exception of the OS rate (Figure 4K), the serious complications group experienced the same DFS rate (Figure 4L).
Gastric cancer is a prevalent type of tumor with consistently high morbidity and mortality rates worldwide[12]. Robotic gastric cancer surgery has progressed rapidly in recent years, and its future prospects continue to attract attention. Robotic surgery has obvious technical advantages and superior efficacy compared with conventional laparoscopy[13]. The progression and prognosis of patients with stomach cancer are influenced by various contributing conditions, with inflammation being an important contributor[14]. The prolonged presence of inflammation may harm the gastric mucosa and cause aberrant cellular proliferation[14]. The activation of the inflammatory response creates favorable for the proliferation and colonization of remnant tumor cells, which promotes the local recurrence and distant metastasis of cancer and reduces patient survival[15]. SIRI, an indicator used for assessing a patient's inflammatory status by integrating multiple inflammatory cells values, has certain advantages and prospects for application. However, the accuracy and reliability of utilizing the SIRI require further research and validation. Therefore, we conducted a meta-analysis to examine the significance of SIRI values in predicting the prognoses of gastric cancer patients and investigated its predictive potential for oncological survival benefits in patients undergoing robotic gastric cancer surgery with the aim of providing clinicians with more comprehensive information that can assist in making more accurate diagnostic and therapeutic decisions.
Tumorigenesis involves the establishment of a preneoplastic inflammatory environment[16]. Neutrophils, as an important immune component of the inflammatory response, have an essential function in tumorigenesis and develo
Monocytes are an integral part of the tumor microenvironment and act on the tumor population through multifarious mechanisms, including the induction of immunological tolerance, angiogenesis and increased tumor cell dissemination[20]. Monocytes are capable of excreting immune suppressants, such as transforming growth factor β and IL-10, which inhibit the sensitivity of immune cells and attenuate the immunological processes helping tumor cells to evade attack by the immune system[21]. Monocytes can differentiate into endothelial cell-like cells that are involved in the tumor angiogenesis process. These cells can produce antiangiogenic agents (vascular endothelial growth factor), which facilitate neovascularization and provide sufficient nutrients and oxygen to the tumor[22]. Monocytes can secrete matrix metalloproteinases, which are enzymes that can degrade the stroma and encourage the invasion and metastasis of the tumor cells[23].
T-cell infiltration that develops in human cancers is a regulator of natural disease progression[24]. Cytotoxic T lym
The SIRI is unique in reflecting the sophisticated interactions and complementary activities of the primary immune cells in the cancer microenvironment. This novel metric is intended for evaluating the survival of patients with malignancies and reflects the state of equilibrium between the immune and inflammatory systems of the host. Increasing clinical studies have reported that SIRI values are strongly associated with the survival time of a wide range of tumor types. Chen et al[28] reported that nasopharyngeal cancer patients with higher SIRI values experienced considerably shorter OS. A separate study reported that postmenopausal women with breast cancer with low SIRI values exhibited notably longer OS[29]. SIRI values demonstrated favorable prognostic ability in a number of solid tumors, including pancreatic, gastric and oesophageal carcinomas[30,31]. The SIRI is suitable for frequent testing during follow-up because it can be conveniently calculated from a hematological count. Both the value and the dynamics of SIRI have the potential to assessing the efficacy of adjuvant radiotherapy, the selection of suitable patients for specific targeted therapies and immunotherapies, and monitoring for possible recurrences. Similarly, SIRI values were associated with OS and DFS in gastric cancer patients in our meta-analysis, providing valid evidence for its potential to predict prognosis. In the population of patients undergoing robotic gastric cancer surgery, SIRI values were also a stable independent prognostic factor for long-term oncological outcomes, with the low SIRI group showing improved survival compared to the high SIRI group. SIRI has the advantages of being easily accessible, with low cost and good reproducibility. SIRI values, using a combination of multiple metrics, can more accurately assess a patient's inflammatory status and tumor load. The continuous improvement and optimization of robotic gastric cancer surgery will enable it to become the mainstream of minimally invasive gastric cancer surgery, and our findings make SIRI an encouraging tool to add credibility to decision-making regarding cancer treatment strategies.
Our study had the following limitations: (1) This study was conducted retrospectively. Thus, a prospective investigation with a large sample size is needed to validate the potential implication of SIRI values in patients undergoing robotic gastric cancer surgery and clarify the optimal SIRI cutoff value; (2) the study did not dynamically assess changes in SIRI values; and (3) the mechanisms associated with SIRI values and cancer prognosis prediction remain unclear and require in-depth studies and confirmation.
Overall, the conclusion of our meta-analysis supports an intrinsic link between SIRI levels and the prognosis of patients with gastric cancer. The findings of our cohort study showed that preoperative SIRI values independently contributed to the OS and DFS in patients who underwent robotic surgery for stomach cancer. The evaluation of SIRI levels can help surgeons and oncologists to more effectively manage preoperative treatment more effectively and develop postoperative management strategies for gastric cancer patients.
Gastric cancer is a prevalent type of tumor with consistently high morbidity and mortality rates worldwide. Robotic gastric cancer surgery has progressed rapidly in recent years, and its future prospects continue to attract attention. Robotic surgery has obvious technical advantages and superior efficacy compared with conventional laparoscopy. The progression and prognosis of patients with stomach cancer are influenced by various contributing conditions, with inflammation being an important contributor. The prolonged presence of inflammation may harm the gastric cancer and cause aberrant cellular proliferation. The activation of the inflammatory response creates favorable for the proliferation and colonization of remnant tumor cells, which promotes the local recurrence and distant metastasis of cancer and reduces patient survival. Systemic inflammatory response index (SIRI), an indicator used for assessing a patient's inflammatory status by integrating multiple inflammatory cells values, has certain advantages and prospects for application.
The accuracy and reliability of using the SIRI need further research and validation. Therefore, we performed a meta-analysis to examine the relevance of SIRI values to the prognoses of gastric cancer patients and investigated its predictive value for oncological survival benefits in patients undergoing robotic gastric cancer surgery with the aim of providing clinicians with more comprehensive information that can assist in making more accurate diagnostic and therapeutic decisions.
The main objective of this study was to examine the relevance of SIRI values to the prognoses of gastric cancer patients and investigate its predictive value for oncological survival benefits in patients undergoing robotic gastric cancer surgery. Our findings make SIRI an encouraging tool to add credibility to decision-making regarding cancer treatment strategies.
We performed a meta-analysis to examine the correlation between SIRI values and the prognosis of patients with gastric cancer and investigated its predictive value for oncological survival benefit in patients who underwent robotic gastric cancer surgery by analyzing data from a retrospective cohort.
Overall, the conclusion of our meta-analysis supports an intrinsic link between SIRI levels and the prognosis of patients with gastric cancer. The findings of our cohort study showed that preoperative SIRI values independently contributed to the overall survival and disease-free survival in patients who underwent robotic surgery for stomach cancer.
The SIRI is unique in reflecting the sophisticated interactions and complementary activities of the major immune cells in the cancer microenvironment. This new metric is designed to assess survival in patients with malignancy and reflects the state of equilibrium between the immune and inflammatory systems of the host. Our findings make SIRI an encouraging tool to add credibility to decision-making regarding cancer treatment strategies.
A prospective investigation with a large sample size is needed to validate the potential implication of SIRI values in patients undergoing robotic gastric cancer surgery and clarify the optimal SIRI cutoff value. The mechanisms associated with SIRI values and cancer prognosis prediction remain unclear and require in-depth studies and confirmation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tovo CV, Brazil S-Editor: Qu XL L-Editor: A P-Editor: Zhang YL
| 1. | Alsina M, Arrazubi V, Diez M, Tabernero J. Current developments in gastric cancer: from molecular profiling to treatment strategy. Nat Rev Gastroenterol Hepatol. 2023;20:155-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 59] [Article Influence: 59.0] [Reference Citation Analysis (1)] |
| 2. | Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. 2023;20:338-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 58] [Reference Citation Analysis (0)] |
| 3. | Terashima M, Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Miki Y, Makuuchi R, Honda S, Tatsubayashi T, Takagi W, Omori H, Hirata F. Robotic surgery for gastric cancer. Gastric Cancer. 2015;18:449-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Beyer K. Surgery Matters: Progress in Surgical Management of Gastric Cancer. Curr Treat Options Oncol. 2023;24:108-129. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 5. | Yang H, Wei B, Hu B. Chronic inflammation and long-lasting changes in the gastric mucosa after Helicobacter pylori infection involved in gastric cancer. Inflamm Res. 2021;70:1015-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Eto K, Hiki N, Kumagai K, Shoji Y, Tsuda Y, Kano Y, Yasufuku I, Okumura Y, Tsujiura M, Ida S, Nunobe S, Ohashi M, Sano T, Yamaguchi T. Prophylactic effect of neoadjuvant chemotherapy in gastric cancer patients with postoperative complications. Gastric Cancer. 2018;21:703-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1802] [Cited by in F6Publishing: 1962] [Article Influence: 130.8] [Reference Citation Analysis (0)] |
| 8. | Song M, Zhang Q, Song C, Liu T, Zhang X, Ruan G, Tang M, Xie H, Zhang H, Ge Y, Li X, Zhang K, Yang M, Li Q, Liu X, Lin S, Xu Y, Xu H, Wang K, Li W, Shi H. The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer. J Cachexia Sarcopenia Muscle. 2022;13:2504-2514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Cui S, Cao S, Chen Q, He Q, Lang R. Preoperative systemic inflammatory response index predicts the prognosis of patients with hepatocellular carcinoma after liver transplantation. Front Immunol. 2023;14:1118053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 10. | Li L, Li Z, Feng X, Yang Z, Jin N, Zhu L, Zang X, Xing L, Ren Y, Zhang H. Predictive value of systemic inflammatory response-related indices for survival in tongue cancer. Oral Dis. 2022;. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 11. | Ye K, Xiao M, Li Z, He K, Wang J, Zhu L, Xiong W, Zhong Z, Tang Y. Preoperative systemic inflammation response index is an independent prognostic marker for BCG immunotherapy in patients with non-muscle-invasive bladder cancer. Cancer Med. 2023;12:4206-4217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50630] [Cited by in F6Publishing: 45365] [Article Influence: 15121.7] [Reference Citation Analysis (47)] |
| 13. | Tian Y, Cao S, Kong Y, Shen S, Niu Z, Zhang J, Chen D, Jiang H, Lv L, Liu X, Li Z, Zhong H, Zhou Y. Short- and long-term comparison of robotic and laparoscopic gastrectomy for gastric cancer by the same surgical team: a propensity score matching analysis. Surg Endosc. 2022;36:185-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Rihawi K, Ricci AD, Rizzo A, Brocchi S, Marasco G, Pastore LV, Llimpe FLR, Golfieri R, Renzulli M. Tumor-Associated Macrophages and Inflammatory Microenvironment in Gastric Cancer: Novel Translational Implications. Int J Mol Sci. 2021;22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 15. | Zhi X, Kuang X, Li J. The Impact of Perioperative Events on Cancer Recurrence and Metastasis in Patients after Radical Gastrectomy: A Review. Cancers (Basel). 2022;14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 16. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10123] [Cited by in F6Publishing: 10649] [Article Influence: 484.0] [Reference Citation Analysis (0)] |
| 17. | Canli Ö, Nicolas AM, Gupta J, Finkelmeier F, Goncharova O, Pesic M, Neumann T, Horst D, Löwer M, Sahin U, Greten FR. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell. 2017;32:869-883.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 18. | 18 Bens S, Mohn A, Yüksel B, Kulle AE, Michalek M, Chiarelli F, Nuri Ozbek M, Leuschner I, Grötzinger J, Holterhus PM, Riepe FG. Congenital lipoid adrenal hyperplasia: functional characterization of three novel mutations in the STAR gene. J Clin Endocrinol Metab. 2010;95:1301-1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, Beisel C, Kurzeder C, Heinzelmann-Schwarz V, Rochlitz C, Weber WP, Beerenwinkel N, Aceto N. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 501] [Cited by in F6Publishing: 684] [Article Influence: 136.8] [Reference Citation Analysis (0)] |
| 20. | Ugel S, Canè S, De Sanctis F, Bronte V. Monocytes in the Tumor Microenvironment. Annu Rev Pathol. 2021;16:93-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 21. | Thepmalee C, Panya A, Sujjitjoon J, Sawasdee N, Poungvarin N, Junking M, Yenchitsomanus PT. Suppression of TGF-β and IL-10 receptors on self-differentiated dendritic cells by short-hairpin RNAs enhanced activation of effector T-cells against cholangiocarcinoma cells. Hum Vaccin Immunother. 2020;16:2318-2327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Arderiu G, Espinosa S, Peña E, Crespo J, Aledo R, Bogdanov VY, Badimon L. Tissue factor variants induce monocyte transformation and transdifferentiation into endothelial cell-like cells. J Thromb Haemost. 2017;15:1689-1703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Sciarra A. Words of wisdom. Re: Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Stillebroer AB, Mulders PFA, Boerman DC, Oyen WJG, Oosterwijk. E Eur Urol. In press. DOI: 10.1016/j.eururo.2010.03.015. Eur Urol. 2010;58:316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | van der Leun AM, Thommen DS, Schumacher TN. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer. 2020;20:218-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 676] [Article Influence: 169.0] [Reference Citation Analysis (0)] |
| 25. | Tsyklauri O, Chadimova T, Niederlova V, Kovarova J, Michalik J, Malatova I, Janusova S, Ivashchenko O, Rossez H, Drobek A, Vecerova H, Galati V, Kovar M, Stepanek O. Regulatory T cells suppress the formation of potent KLRK1 and IL-7R expressing effector CD8 T cells by limiting IL-2. Elife. 2023;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 26. | Lhuillier C, Rudqvist NP, Yamazaki T, Zhang T, Charpentier M, Galluzzi L, Dephoure N, Clement CC, Santambrogio L, Zhou XK, Formenti SC, Demaria S. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J Clin Invest. 2021;131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 104] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 27. | Kwakkenbos MJ, van Helden PM, Beaumont T, Spits H. Stable long-term cultures of self-renewing B cells and their applications. Immunol Rev. 2016;270:65-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Chen Y, Jiang W, Xi D, Chen J, Xu G, Yin W, Gu W. Development and validation of nomogram based on SIRI for predicting the clinical outcome in patients with nasopharyngeal carcinomas. J Investig Med. 2019;67:691-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Hua X, Long ZQ, Huang X, Deng JP, Wen W, He ZY, Guo L, Zhang WW, Lin HX. The preoperative systemic inflammation response index (SIRI) independently predicts survival in postmenopausal women with breast cancer. Curr Probl Cancer. 2020;44:100560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Li S, Lan X, Gao H, Li Z, Chen L, Wang W, Song S, Wang Y, Li C, Zhang H, Xue Y. Systemic Inflammation Response Index (SIRI), cancer stem cells and survival of localised gastric adenocarcinoma after curative resection. J Cancer Res Clin Oncol. 2017;143:2455-2468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Geng Y, Zhu D, Wu C, Wu J, Wang Q, Li R, Jiang J. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with esophageal squamous cell carcinoma. Int Immunopharmacol. 2018;65:503-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |