Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2757
Peer-review started: September 14, 2023
First decision: October 8, 2023
Revised: October 23, 2023
Accepted: November 25, 2023
Article in press: November 25, 2023
Published online: December 27, 2023
Peptic ulcer (PU) is an abnormal phenomenon in which there is rupture of the mucosa of the digestive tract, which not only affects patients' normal life but also causes an economic burden due to its high medical costs.
To investigate the efficacy of pantoprazole (PPZ) plus perforation repair in patients with PU and its effect on the stress response.
The study subjects were 108 PU patients admitted between July 2018 and July 2022, including 58 patients receiving PPZ plus perforation repair [research group (RG)] and 50 patients given simple perforation repair [control group (CG)]. The efficacy, somatostatin (SS) concentration, stress reaction [malondialdehyde (MDA), lipid peroxide (LPO)], inflammatory indices [tumor necrosis factor (TNF)-α, C-reactive protein (CRP), interleukin (IL)-1β], recurrence, and complications (perforation, hemorrhage, and pyloric obstruction) were compared.
The overall response rate was higher in the RG than in the CG. Patients in the RG had markedly elevated SS after treatment, which was higher than that of the CG, while MDA, LPO, TNF-, CRP, and IL-1β were significantly reduced to lower levels than those in the CG. Lower recurrence and complication rates were identified in the RG group.
Therefore, PPZ plus perforation repair is conducive to enhancing treatment outcomes in PU patients, reducing oxidative stress injury and excessive inflammatory reactions, and contributing to low recurrence and complication rates.
Core Tip: Peptic ulcer (PU), as a chronic disease, may cause complications such as perforation, upper gastrointestinal bleeding, and rarely gastric outlet obstruction. Risk factors such as advanced age, a history of PU, Helicobacter pylori infection, and the use of nonsteroidal anti-inflammatory drugs further increase the risk of developing the disease. To further reduce the associated negative effects of the disease, this study aims to explore and seek new therapeutic models to improve the management of the disease.
- Citation: Leng ZY, Wang JH, Gao L, Shi K, Hua HB. Efficacy of pantoprazole plus perforation repair for peptic ulcer and its effect on the stress response. World J Gastrointest Surg 2023; 15(12): 2757-2764
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2757.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2757
Peptic ulcer (PU), as a disease of the digestive system, is essentially a phenomenon of digestive tract mucosal rupture, which mainly appears in the stomach and duodenum[1,2]. The disease may cause complications such as perforation, upper gastrointestinal bleeding, and rarely gastric outlet obstruction, which poses a threat to the health of patients[3,4]. Risk factors for PU include advanced age, history of PU, Helicobacter pylori infection, and use of nonsteroidal anti-inflammatory drugs[5]. The disease may lead to gastrointestinal symptoms such as epigastric pain, burping, vomiting and heartburn, hindering the patient’s normal life activities and lowering the quality of life[6]. According to statistics, the lifetime risk of PU can be as high as 10%, with at least 4 million people affected by the disease every year, which causes these patients to have high medical costs[7,8]. To reduce the negative effects of PU, this study seeks new modalities of treatment to improve the management of the disease.
Perforation repair, a surgical procedure used to prevent the recurrence of ulcers in PU, has the advantages of minimal invasiveness, low surgical difficulty, low surgical risk and little influence on patients' abdominal organs[9,10]. In the study by Varcus et al[11], perforation repair was more effective than open repair in accelerating the recovery of patients with PU perforation and was associated with a lower risk of morbidity and mortality, suggesting that the former has a clinical advantage over the latter in the treatment of PU. Kim et al[12] also reported that perforation repair is more beneficial to digestive tract function recovery and can play a therapeutic role in hemodynamically unstable patients while ensuring a good level of safety. Pantoprazole (PPZ), on the other hand, is a proton pump inhibitor that can affect the structure and function of the gastric mucosa and reduce the acid secretion of gastric parietal cells, helping alleviate diseases such as heartburn, gastroesophageal reflux disease and PU[13,14]. In the research of Moayyedi et al[15], PPZ had a preventive effect on gastroduodenal bleeding in patients with stable cardiovascular disease and peripheral arterial disease. PPZ, although long-acting, is less available when taken orally than when intravenously injected, so it is often administered intravenously[16].
This study proposes that PPZ plus perforation repair has a better therapeutic effect and clinical application value than perforation repair alone in PU patients, and the results validated this hypothesis.
The eligibility criteria were as follows: A diagnosis of PU[17], first-time treatment for PU, no contraindications to the study medication plans, and complete case data. The exclusion criteria were as follows: Abnormal coagulation function; other gastrointestinal diseases; malignant tumor or severe organ dysfunction; psychiatric disorders or serious infectious diseases; cardiac and renal insufficiency; and pregnancy or lactation. According to the above eligibility and exclusion criteria, 108 PU patients were deemed eligible, whose treatment time was from July 2018 to July 2022. As detailed in Table 1, the general data, such as sex and age, of the two groups were clinically comparable (P > 0.05).
| Indicators | Control group (n = 50) | Research group (n = 58) | χ2/t | P value |
| Sex (male/female) | 29/21 | 35/23 | 0.061 | 0.805 |
| Age (yr) | 49.10 ± 8.08 | 48.50 ± 7.21 | ||
| Perforation site (stomach/duodenum/mixed) | 22/20/8 | 20/27/11 | 1.025 | 0.599 |
| History of alcoholism (with/without) | 16/34 | 20/38 | 0.074 | 0.785 |
| History of smoking (yes/no) | 13/37 | 13/45 | 0.189 | 0.664 |
Perforation repair: Each patient underwent endotracheal intubation for general anesthesia, and pneumoperitoneum was established using the three-hole technique, followed by placement of the remaining sheath catheters. Abdominal exploration was performed to further rule out gastric cancer after drainage of the abdominal fluid and food debris. After feeding 0-3 absorbable surgical sutures through the main operation hole with a needle, the Scanlan needle holder was used to tie a knot in the cavity according to the conventional upper digestive tract perforation repair method. After 2-4 stitches, the fixed part of the omentum was covered at the perforation suture. The epiploic foramen was drained following irrigation of the abdominal cavity, and the abdomen was closed.
Regarding the administration of PPZ, 40 mg of PPZ in 0.9% normal saline was injected as an intravenous drip during postoperative fasting once daily for 3-5 d. PPZ (40 mg) was given twice a day after the initiation of postoperative oral food intake, which was supplemented by colloidal bismuth pectin capsules (100 mg) three times a day, for 6-8 wk.
The research group (RG) received PPZ plus perforation repair, while the control group (CG) received perforation repair alone.
Efficacy: If the patient's ulcer surface was basically healed with no inflammation around it, it was considered to be cured. A marked response was indicated by the disappearance of the ulcer surface and some inflammation around it. An improvement referred to an ulcer area reduction of less than 50.0%. Nonresponse referred to no change or even worsening of the ulcer surface.
Somatostatin (SS) and stress response: Five milliliters of fasting venous blood was collected in the early morning before and after treatment, and the serum was separated by centrifugation to determine stress-related indices such as SS, malondialdehyde (MDA) and lipid peroxide (LPO) using immunoturbidimetry.
Inflammation indices: Enzyme-linked immunosorbent assays were carried out to measure tumor necrosis factor (TNF)-α, C-reactive protein (CRP) and interleukin (IL)-1β levels in strict accordance with the kit instructions.
Recurrence: Recurrent cases were counted, from which the recurrence rate was calculated.
Occurrence of complications: We observed and counted the number of patients with complications, such as perforation, bleeding, and pyloric obstruction, and calculated the overall incidence.
In this study, both continuous (represented by mean ± SEM) and categorical variables [denoted by n (%)] were imported into GraphPad Prism 7.0 for statistical analysis and graph drawing. To identify intergroup differences, the t test and the χ2 test were used for continuous and categorical variables, respectively. All analyses relied upon a P < 0.05 statistical significance criterion.
The RG and CG had similar general data, such as sex, age, lesion site, alcohol abuse history, and smoking history (P > 0.05, Table 1).
The overall response rates of the RG and CG were 89.66% (37 cases) and 74.00% (52 cases), respectively. The above data revealed a markedly higher overall response rate in the RG than in the CG (P < 0.05, Table 2).
| Indicators | Control group (n = 50) | Research group (n = 58) | χ2 | P value |
| Marked response | 22 (44.00) | 30 (51.72) | ||
| Response | 15 (30.00) | 22 (37.93) | ||
| Nonresponse | 13 (26.00) | 6 (10.34) | ||
| Overall response | 37 (74.00) | 52 (89.66) | 4.539 | 0.033 |
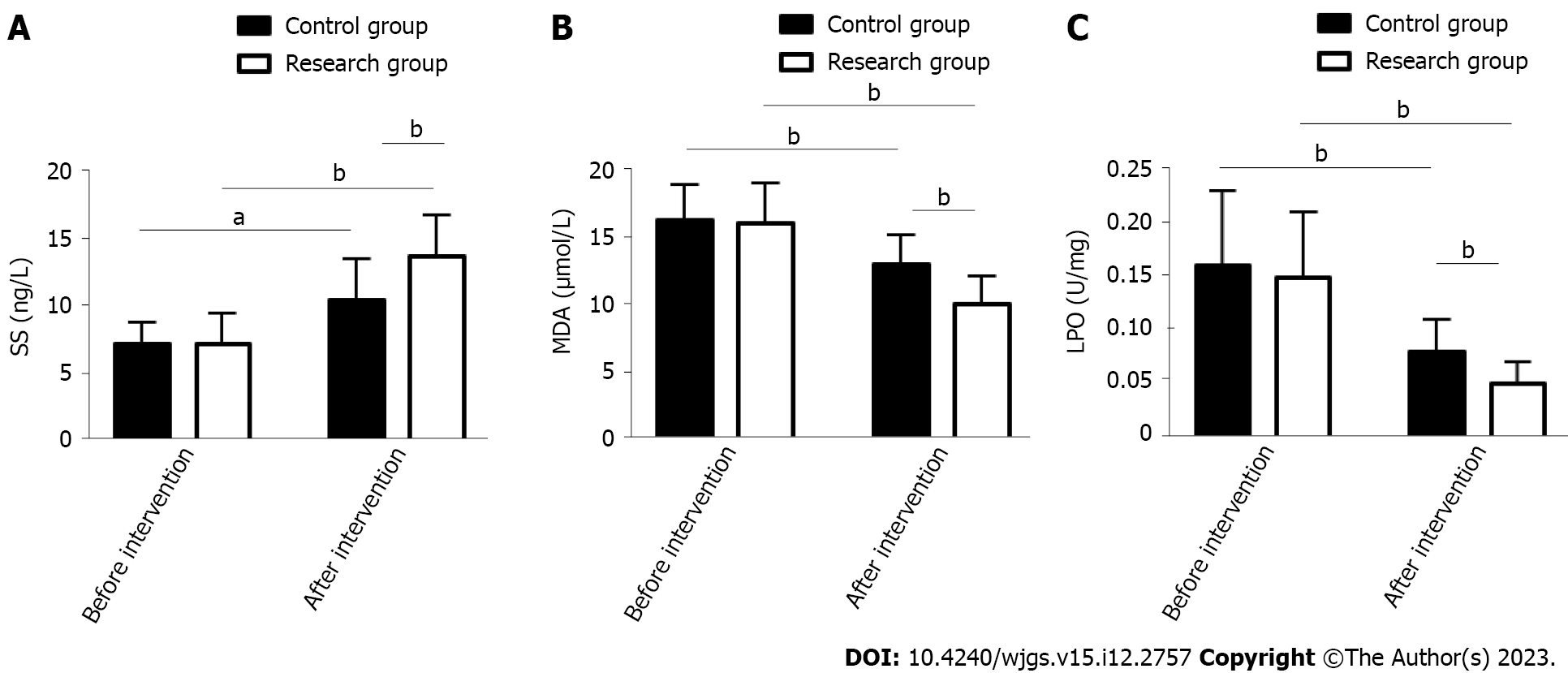
The effects of the two treatment schemes on the stress response of PU patients were evaluated by measuring stress response indices such as SS, MDA and LPO. The above indices were similar between the groups prior to treatment (P > 0.05). In both groups, SS was elevated and MDA and LPO were reduced after treatment, but SS was even higher and MDA and LPO were even lower in the RG (P < 0.05) (Figure 1).
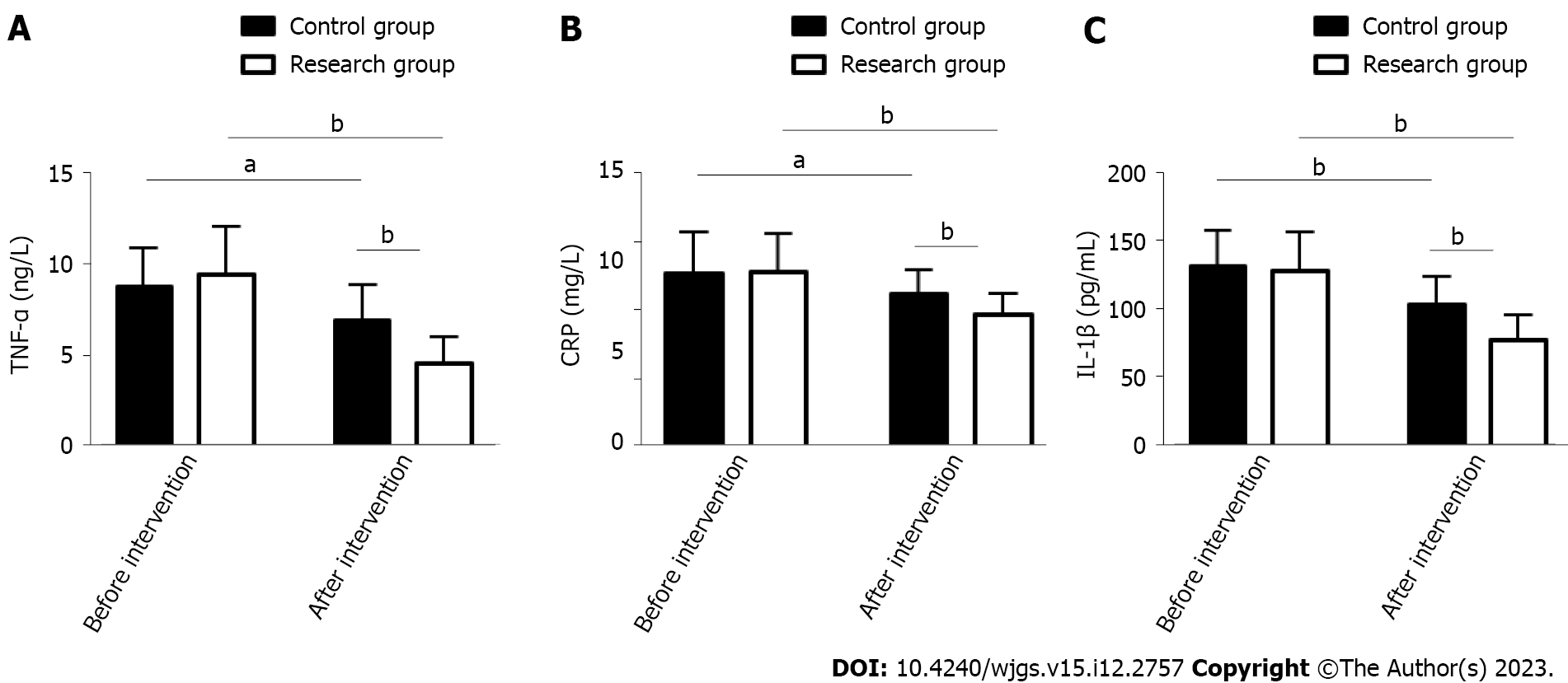
The inflammatory indicators TNF-α, CRP, and IL-1β were measured to evaluate the effects of the two treatment schemes on inflammation in PU patients. As above, these indices were similar between the groups prior to treatment (P > 0.05). An evident reduction was observed in TNF-, CRP, and IL-1β in both groups after treatment (P < 0.05), which were more extreme in the RG vs CG (P < 0.05, Figure 2).
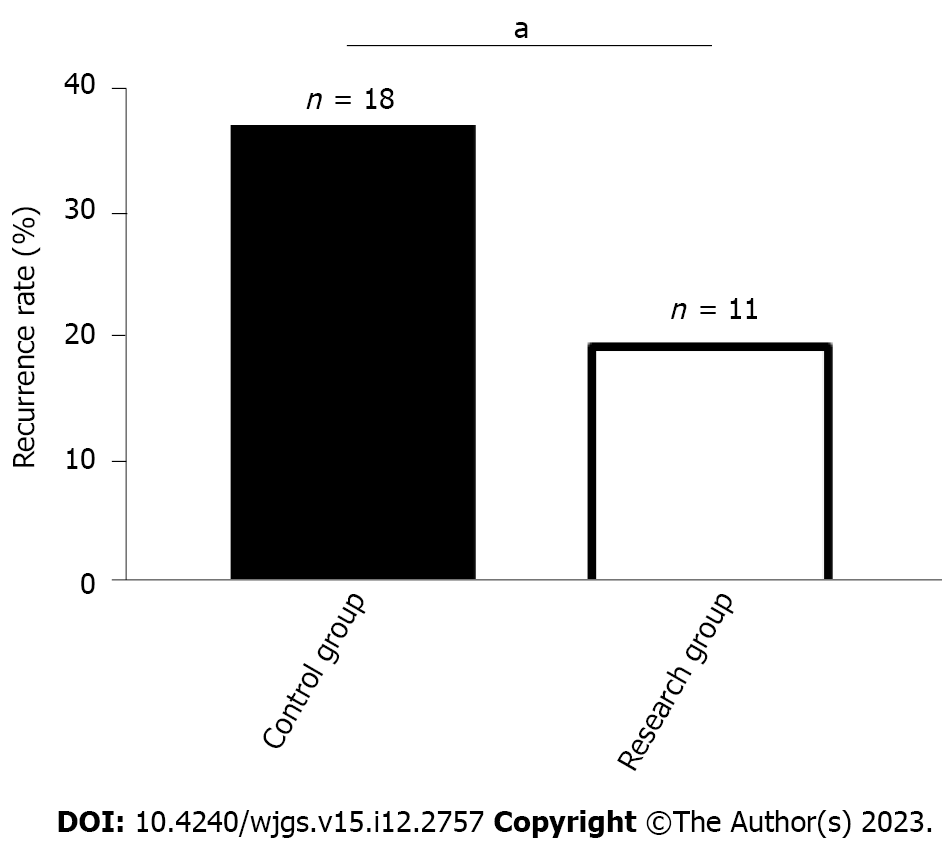
The number of recurrent cases in the RG and CG was 11 (18.97%) and 18 (36.00%), respectively, which was a lower recurrence rate in the RG than in the CG (P < 0.05, Figure 3).
Both groups of patients suffered from perforation, bleeding, and pyloric obstruction, with overall incidences of 12.00% and 0.00% in the CG and RG, respectively. When comparing the groups, the overall complication rate was markedly lower in the RG than in the CG (P < 0.05, Table 3).
| Indicators | Control group (n = 50) | Research group (n = 58) | χ2 | P value |
| Perforation | 0 (0.00) | 0 (0.00) | ||
| Hemorrhage | 3 (6.00) | 0 (0.00) | ||
| Pyloric obstruction | 3 (6.00) | 0 (0.00) | ||
| Total | 6 (12.00) | 0 (0.00) | 7.369 | 0.007 |
PU is a chronic disease. Although its etiology remains unclear, PU is known to be pathogenically associated with the weakening of the gastric mucosal protection mechanism and with excessive gastric acid[18]. The risk of perforation in patients with this disease can be as high as 14%. PU perforation generally manifests as diffuse peritonitis and systemic septicemia, which may endanger patients' lives[19,20]. This study proposes a therapeutic regimen to treat patients with PU perforation in the search for effective treatment options and in the interest of providing a new clinical reference.
This study included 108 PU patients and grouped them by treatment scheme: Those receiving PPZ plus perforation repair and those treated by perforation repair alone were assigned to the RG and CG, respectively. In our study, the overall response rate of the RG was significantly higher than that of the CG (89.66% vs 74.00%), indicating that PPZ plus perforation repair for PUs can help improve efficacy, with better efficacy than perforation repair alone. Previous studies have pointed out that although surgical repair is the standard of treatment for most PU patients, its combination with proton pump inhibitors may be considered because the combination therapy may not only help reduce surgery-related risk but also limit the abuse of proton pump inhibitors, so the two have a certain synergistic role in treatment[21,22]. Tan et al[23] pointed out that although perforation repair has the same therapeutic effect as open surgery, the former has the advantages of a lower surgical site infection rate, less postoperative pain, and a shorter nasogastric tube use time. On the other hand, SS has a positive effect on the healing of PU, which may be related to its inhibition of gastrin secretion[24]. Both MDA and LPO can reflect oxidative stress injury associated with PU; the abnormal upregulation of the former is related to the aggravation of cell membrane damage, and the abnormal secretion of the latter is closely related to the deterioration of gastric mucosal tissue damage[25,26]. Therefore, stress response indices such as SS, MDA and LPO were measured to evaluate the ulcer healing and stress response performance of our PU patients. We found that after treatment, RG had a posttreatment SS level that was significantly higher than the pretreatment level and significantly higher than the CG posttreatment level, while MDA and LPO were significantly lower, suggesting that PPZ plus perforation repair can significantly promote ulcer healing and reduce the stress response. The RG had markedly lower inflammatory indices than the pretreatment levels and the CG levels, indicating that PPZ plus perforation repair can effectively inhibit inflammation in PU patients. Recurrence was rarer in the RG vs CG (18.97% vs 36.00%), suggesting that PPZ plus perforation repair reduces the recurrence risk of PU. In the study by Ng et al[27], PPZ was superior to high-dose famotidine in preventing the recurrence of aspirin-related PU, suggesting that PPZ can help to reduce the recurrence risk of PU patients, similar to our findings. Our RG had a markedly lower overall incidence of complications such as perforation, bleeding and pyloric obstruction than the CG (0.00% vs 12.00%), demonstrating that PPZ plus perforation repair can help prevent the risk of complications to some extent. Tulinský et al[28] observed that perforation repair for patients with PU perforation was superior to traditional open surgery in terms of safety, and the former helped reduce the incidence of postoperative complications and mortality to some extent, which is consistent with our results.
There are several limitations of this study that need to be addressed. First, since the sample size of this study is only 108, it is necessary to increase the sample size to improve the accuracy of the results. Second, follow-up data were not included to analyze the long-term efficacy and prognosis of PPZ plus perforation repair in PU patients. Supplementing this study with relevant analyses in this regard will help to further demonstrate the potential clinical advantages of PPZ plus perforation repair. Third, this study is a single-center study, and if it can be expanded into a multicenter study, information collection bias can be avoided to a certain extent. In the future, supplementary analyses of the above three areas for improvement will be carried out gradually.
In summary, PPZ plus perforation repair is effective in treating PU, as it can suppress the stress response by downregulating MDA and LPO and upregulating SS and can inhibit the inflammatory response by downregulating TNF-α, CRP and IL-1β while reducing the risks of recurrence and complications. Our findings provide a new theoretical basis for the prevention and treatment of PU patients, as well as provides a novel treatment choice for PU management.
Peptic ulcer (PU) is an abnormal phenomenon of rupture of the mucosa of the digestive tract, which not only affects patients' normal life but also causes an economic burden due to its high medical costs.
There is an urgent need to improve the management of PU from the treatment model and to provide effective treatment options and new clinical references for patients with the disease.
This study investigated the efficacy of pantoprazole (PPZ) plus perforation repair in patients with PU and its effect on the stress response.
The study subjects were 108 PU patients admitted between July 2018 and July 2022, including 58 patients receiving PPZ plus perforation repair [research group (RG)] and 50 patients given simple perforation repair [control group (CG)]. The efficacy, somatostatin (SS) concentration, stress reaction [malondialdehyde (MDA), lipid peroxide (LPO)], inflammatory indices [tumor necrosis factor (TNF)-α, C-reactive protein (CRP), interleukin (IL)-1β], recurrence, and complications (perforation, hemorrhage, and pyloric obstruction) were compared.
The overall response rate was higher in RG than in CG. RG had markedly elevated SS after treatment, which was higher than that of CG, while MDA, LPO, TNF-α, CRP, and IL-1β were significantly reduced to lower than those in CG. Lower recurrence and complication rates were identified in RG.
Therefore, PPZ plus perforation repair is conducive to enhancing treatment outcomes in PU patients, reducing oxidative stress injury and excessive inflammatory reactions, and contributing to low recurrence and complication rates.
PPZ plus perforation repair is effective in the treatment of PU patients, which can inhibit stress response by down-regulating MDA and LPO and up-regulating SS, alleviate inflammation by down-regulating TNF-α, CRP and IL-1β levels, and help reduce the risk of recurrence and complications. Our paper develops a theoretical foundation for the prevention and treatment of PU patients and provides a new treatment option and direction for the management of the disease.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ermolao A, Italy; Ren-Fielding CJ, United States; Brisinda G, Italy S-Editor: Lin C L-Editor: A P-Editor: Lin C
| 1. | Greenwood-Van Meerveld B, Johnson AC, Grundy D. Gastrointestinal Physiology and Function. Handb Exp Pharmacol. 2017;239:1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Ardalani H, Hadipanah A, Sahebkar A. Medicinal Plants in the Treatment of Peptic Ulcer Disease: A Review. Mini Rev Med Chem. 2020;20:662-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 434] [Article Influence: 62.0] [Reference Citation Analysis (2)] |
| 4. | Shim YK, Kim N. Nonsteroidal Anti-inflammatory Drug and Aspirin-induced Peptic Ulcer Disease. Korean J Gastroenterol. 2016;67:300-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Melcarne L, García-Iglesias P, Calvet X. Management of NSAID-associated peptic ulcer disease. Expert Rev Gastroenterol Hepatol. 2016;10:723-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Kavitt RT, Lipowska AM, Anyane-Yeboa A, Gralnek IM. Diagnosis and Treatment of Peptic Ulcer Disease. Am J Med. 2019;132:447-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 7. | Chan KS, Wang YL, Chan XW, Shelat VG. Outcomes of omental patch repair in large or giant perforated peptic ulcer are comparable to gastrectomy. Eur J Trauma Emerg Surg. 2021;47:1745-1752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Chung KT, Shelat VG. Perforated peptic ulcer - an update. World J Gastrointest Surg. 2017;9:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 127] [Cited by in F6Publishing: 112] [Article Influence: 16.0] [Reference Citation Analysis (4)] |
| 9. | Antoniou SA, Antoniou GA, Koch OO, Pointner R, Granderath FA. Meta-analysis of laparoscopic versus open repair of perforated peptic ulcer. JSLS. 2013;17:15-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Siow SL, Mahendran HA, Wong CM, Hardin M, Luk TL. Laparoscopic versus open repair of perforated peptic ulcer: Improving outcomes utilizing a standardized technique. Asian J Surg. 2018;41:136-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Varcus F, Paun I, Duta C, Dobrescu A, Frandes M, Tarta C. Laparoscopic repair of perforated peptic ulcer. Minerva Chir. 2018;73:188-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Kim CW, Kim JW, Yoon SN, Oh BY, Kang BM. Laparoscopic repair of perforated peptic ulcer: a multicenter, propensity score matching analysis. BMC Surg. 2022;22:230. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 13. | Assalin HB, De Almeida KCG, Guadagnini D, Santos A, Teixeira CJ, Bordin S, Rocha GZ, Saad MJA. Proton Pump Inhibitor Pantoprazole Modulates Intestinal Microbiota and Induces TLR4 Signaling and Fibrosis in Mouse Liver. Int J Mol Sci. 2022;23. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 14. | Asdaq SMB, Swathi E, Dhamanigi SS, Asad M, Ali Mohzari Y, Alrashed AA, Alotaibi AS, Mohammed Alhassan B, Nagaraja S. Role of Daucus carota in Enhancing Antiulcer Profile of Pantoprazole in Experimental Animals. Molecules. 2020;25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Moayyedi P, Eikelboom JW, Bosch J, Connolly SJ, Dyal L, Shestakovska O, Leong D, Anand SS, Störk S, Branch KRH, Bhatt DL, Verhamme PB, O'Donnell M, Maggioni AP, Lonn EM, Piegas LS, Ertl G, Keltai M, Cook Bruns N, Muehlhofer E, Dagenais GR, Kim JH, Hori M, Steg PG, Hart RG, Diaz R, Alings M, Widimsky P, Avezum A, Probstfield J, Zhu J, Liang Y, Lopez-Jaramillo P, Kakkar A, Parkhomenko AN, Ryden L, Pogosova N, Dans A, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik T, Vinereanu D, Tonkin AM, Lewis BS, Felix C, Yusoff K, Metsarinne K, Fox KAA, Yusuf S; COMPASS Investigators. Pantoprazole to Prevent Gastroduodenal Events in Patients Receiving Rivaroxaban and/or Aspirin in a Randomized, Double-Blind, Placebo-Controlled Trial. Gastroenterology. 2019;157:403-412.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 16. | Karabulut K, Dinçer M, Liman RK, Usta S. Non-operative management of perforated peptic ulcer: A single-center experience. Ulus Travma Acil Cerrahi Derg. 2019;25:585-588. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 17. | Kamada T, Satoh K, Itoh T, Ito M, Iwamoto J, Okimoto T, Kanno T, Sugimoto M, Chiba T, Nomura S, Mieda M, Hiraishi H, Yoshino J, Takagi A, Watanabe S, Koike K. Evidence-based clinical practice guidelines for peptic ulcer disease 2020. J Gastroenterol. 2021;56:303-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 18. | Mihály E, Micsik T, Juhász M, Herszényi L, Tulassay Z. Gastritis and gastropathy. Orv Hetil. 2014;155:43-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Coco D, Leanza S. A Review on Treatment of Perforated Peptic Ulcer by Minimally Invasive Techniques. Maedica (Bucur). 2022;17:692-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 20. | Tarasconi A, Coccolini F, Biffl WL, Tomasoni M, Ansaloni L, Picetti E, Molfino S, Shelat V, Cimbanassi S, Weber DG, Abu-Zidan FM, Campanile FC, Di Saverio S, Baiocchi GL, Casella C, Kelly MD, Kirkpatrick AW, Leppaniemi A, Moore EE, Peitzman A, Fraga GP, Ceresoli M, Maier RV, Wani I, Pattonieri V, Perrone G, Velmahos G, Sugrue M, Sartelli M, Kluger Y, Catena F. Perforated and bleeding peptic ulcer: WSES guidelines. World J Emerg Surg. 2020;15:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 21. | Krag M, Marker S, Perner A, Wetterslev J, Wise MP, Schefold JC, Keus F, Guttormsen AB, Bendel S, Borthwick M, Lange T, Rasmussen BS, Siegemund M, Bundgaard H, Elkmann T, Jensen JV, Nielsen RD, Liboriussen L, Bestle MH, Elkjær JM, Palmqvist DF, Bäcklund M, Laake JH, Bådstøløkken PM, Grönlund J, Breum O, Walli A, Winding R, Iversen S, Jarnvig IL, White JO, Brand B, Madsen MB, Quist L, Thornberg KJ, Møller A, Wiis J, Granholm A, Anthon CT, Meyhoff TS, Hjortrup PB, Aagaard SR, Andreasen JB, Sørensen CA, Haure P, Hauge J, Hollinger A, Scheuzger J, Tuchscherer D, Vuilliomenet T, Takala J, Jakob SM, Vang ML, Pælestik KB, Andersen KLD, van der Horst ICC, Dieperink W, Fjølner J, Kjer CKW, Sølling C, Sølling CG, Karttunen J, Morgan MPG, Sjøbø B, Engstrøm J, Agerholm-Larsen B, Møller MH; SUP-ICU trial group. Pantoprazole in Patients at Risk for Gastrointestinal Bleeding in the ICU. N Engl J Med. 2018;379:2199-2208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 22. | Clarke K, Adler N, Agrawal D, Bhakta D, Sata SS, Singh S, Gupta A, Pahwa A, Pherson E, Sun A, Volpicelli F, Cho HJ. Indications for the Use of Proton Pump Inhibitors for Stress Ulcer Prophylaxis and Peptic Ulcer Bleeding in Hospitalized Patients. Am J Med. 2022;135:313-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Tan S, Wu G, Zhuang Q, Xi Q, Meng Q, Jiang Y, Han Y, Yu C, Yu Z, Li N. Laparoscopic vs open repair for perforated peptic ulcer: A meta analysis of randomized controlled trials. Int J Surg. 2016;33 Pt A:124-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Batista LM, Lima GR, De Almeida AB, Magri Lde P, Calvo TR, Ferreira AL, Pellizzon CH, Hiruma-Lima CA, Vilegas W, Sano PT, Brito AR. Ulcer healing and mechanism(s) of action involved in the gastroprotective activity of fractions obtained from Syngonanthus arthrotrichus and Syngonanthus bisulcatus. BMC Complement Altern Med. 2015;15:391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Pan Z, He Q, Zeng J, Li S, Li M, Chen B, Yang J, Xiao J, Zeng C, Luo H, Wang H. Naringenin protects against iron overload-induced osteoarthritis by suppressing oxidative stress. Phytomedicine. 2022;105:154330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Rovani BT, de Freitas RB, Augusti PR, Araldi IC, Somacal S, Quatrin A, Emanuelli T, da Rocha MP, Bauermann Lde F. Prooxidant activity of norbixin in model of acute gastric ulcer induced by ethanol in rats. Hum Exp Toxicol. 2016;35:737-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Ng FH, Wong SY, Lam KF, Chu WM, Chan P, Ling YH, Kng C, Yuen WC, Lau YK, Kwan A, Wong BC. Famotidine is inferior to pantoprazole in preventing recurrence of aspirin-related peptic ulcers or erosions. Gastroenterology. 2010;138:82-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Tulinský L, Sengul D, Sengul I, Hrubovčák J, Martínek L, Kepičová M, Pelikán A, Ihnát P. Laparoscopic Repair Modality of Perforated Peptic Ulcer: Less Is More? Cureus. 2022;14:e30926. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |