Published online Nov 27, 2023. doi: 10.4240/wjgs.v15.i11.2627
Peer-review started: July 10, 2023
First decision: September 6, 2023
Revised: September 23, 2023
Accepted: October 26, 2023
Article in press: October 26, 2023
Published online: November 27, 2023
The prevalence of multiple primary malignant neoplasms (MPMNs) is increasing in parallel with the incidence of malignancies, the continual improvement of diagnostic models, and the extended life of patients with tumors, especially those of the digestive system. However, the co-existence of MPMNs and duodenal adenocarcinoma (DA) is rarely reported. In addition, there is a lack of comprehensive analysis of MPMNs regarding multi-omics and the tumor microenvironment (TME).
In this article, we report the case of a 56-year-old man who presented with a complaint of chest discomfort and abdominal distension. The patient was diagnosed with metachronous esophageal squamous cell carcinoma and DA in the Department of Oncology. He underwent radical resection and chemotherapy for the esophageal tumor, as well as chemotherapy combined with a programmed death-1 inhibitor for the duodenal tumor. The overall survival was 16.6 mo. Extensive evaluation of the multi-omics and microenvironment features of primary and metastatic tumors was conducted to: (1) Identify the reasons responsible for the poor prognosis and treatment resistance in this case; and (2) Offer novel diagnostic and therapeutic approaches for MPMNs. This case demonstrated that the development of a second malignancy may be independent of the location of the first tumor. Thus, tumor recurrence (including metastases) should be distinguished from the second primary for an accurate diagnosis of MPMNs.
Multi-omics characteristics and the TME may facilitate treatment selection, improve efficacy, and assist in the prediction of prognosis.
Core Tip: Multiple primary malignant neoplasms (MPMNs) are increasingly prevalent in clinical practice, most frequently in the digestive system. We report a rare case of MPMN with a combination of esophageal squamous cell carcinoma and duodenal adenocarcinoma. According to PubMed-indexed literature, there are no standard guidelines or expert consensus on the etiology and comprehensive treatment. We also conducted a detailed study of the features of primary and metastatic tumors. The aim of this report was to identify the reasons responsible for the poor prognosis and treatment resistance in this case through histological data and provide new diagnostic and treatment directions for MPMNs.
- Citation: Huang CC, Ying LQ, Chen YP, Ji M, Zhang L, Liu L. Metachronous primary esophageal squamous cell carcinoma and duodenal adenocarcinoma: A case report and review of literature. World J Gastrointest Surg 2023; 15(11): 2627-2638
- URL: https://www.wjgnet.com/1948-9366/full/v15/i11/2627.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i11.2627
Multiple primary malignant neoplasms (MPMNs), also termed multiple primary cancers, refer to two or more primary tumors that occur simultaneously or sequentially in a single or multiple organs[1]. According to the time interval from the diagnosis of the first tumor, MPMNs are divided into synchronous cancer (SC) (< 6 mo) and metachronous cancer (MC) (≥ 6 mo)[2]. The detection rate of the second or multiple primary tumors is also on the rise due to newer diagnostic methods and treatments, as well as the longer survival times of patients with cancer. MPMNs are most commonly reported in the digestive system; however, their occurrence in combination with duodenal adenocarcinoma (DA) is extremely rare. In this article, we describe the case of a patient who had metachronous esophageal squamous cell carcinoma (ESCC) and DA with multiple metastases. In this analysis, we thoroughly examined the multi-omics features and tumor-related immune microenvironment.
A 56-year-old Chinese man presented to a local hospital with a complaint of chest discomfort and abdominal distension.
The symptoms developed 2 mo before presentation to hospital.
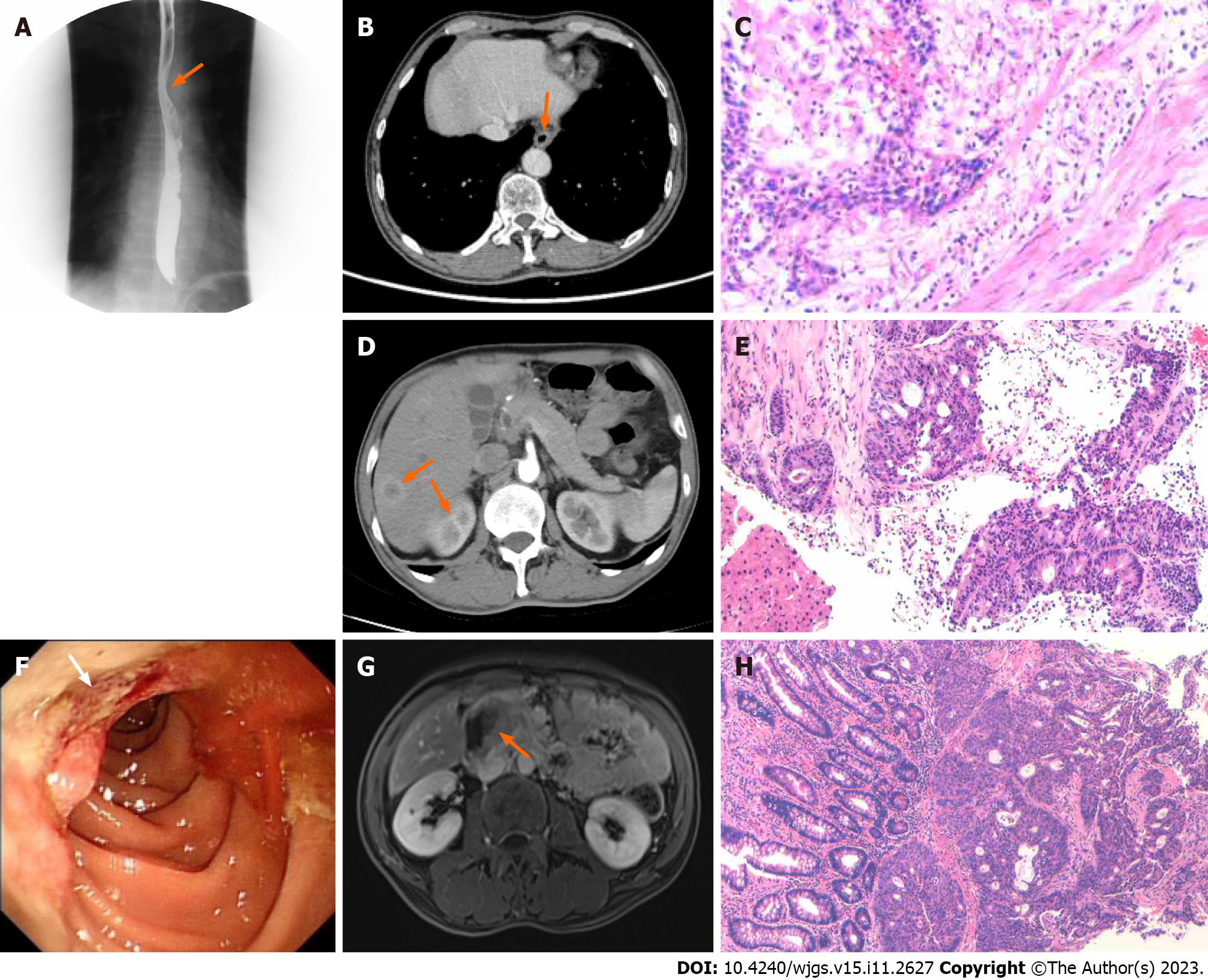
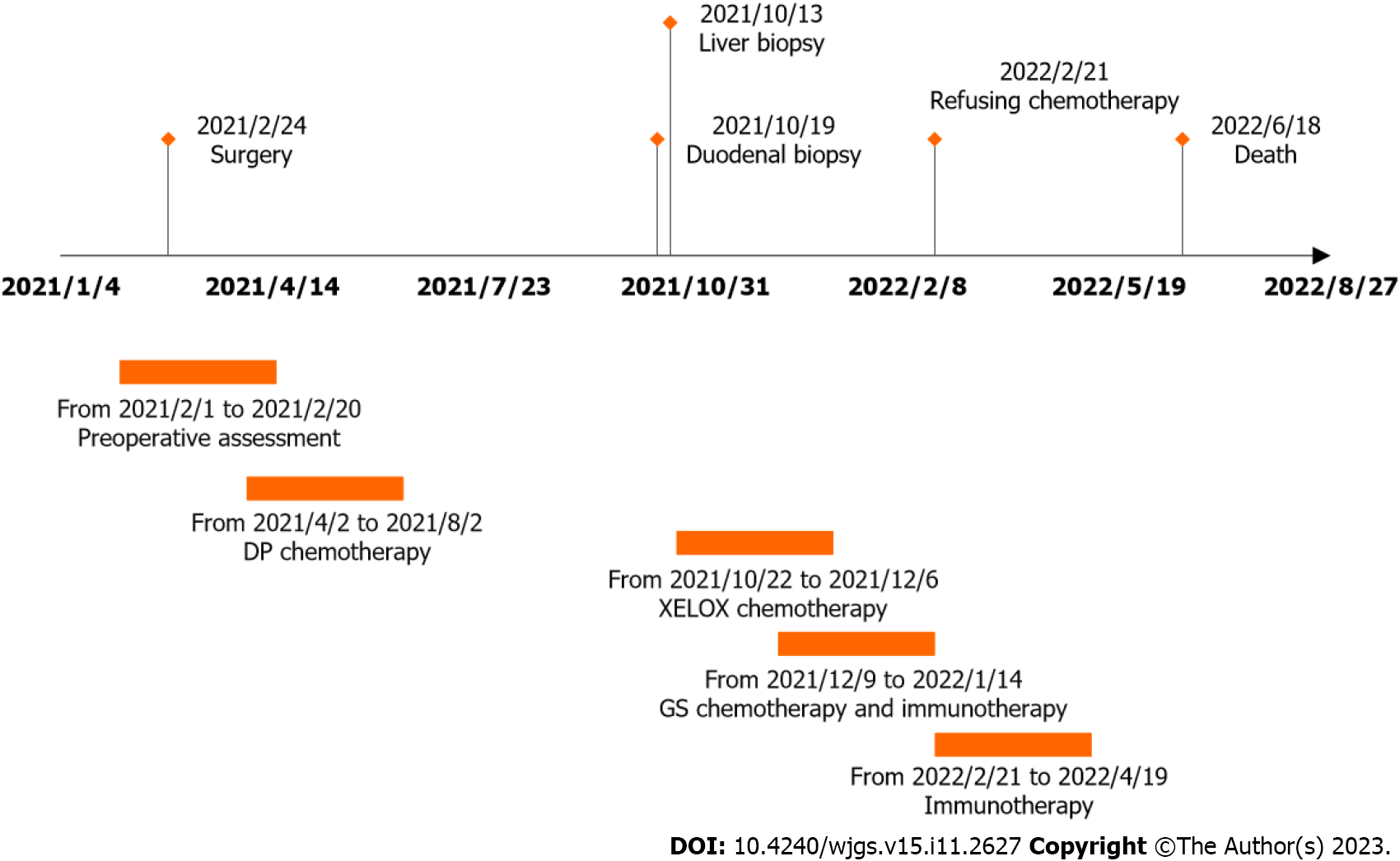
Endoscopic examination, performed on February 1, 2021, revealed the presence of ulcerative lesions in the left wall of the esophagus. These lesions were brittle and prone to bleeding when touched. Frosty ulcers were also detected in the duodenal bulb. Histopathological analysis of the esophagus indicated moderately differentiated squamous cell carcinoma. The patient visited the Thoracic Surgery Department of the General Hospital for further treatment. The preoperative levels of alpha-fetoprotein were 16.6 ng/mL (0-7 ng/mL), whereas those of other gastrointestinal tumor indicators were within the normal range. The upper gastrointestinal tract barium meal revealed a localization in the lower and middle esophagus (Figure 1A). Further evaluation through enhanced computed tomography (CT) of the chest and upper abdomen showed thickening and enhancement of the lower esophagus wall (Figure 1B). A thoracoscopic laparoscopy combined with radical resection of esophageal tumors was carried out on February 24, 2021. Postoperative pathological analysis revealed that the tumor was completely located in the esophagus and did not involve the gastroesophageal junction; the tumor dimensions were 3 cm × 2 cm × 1 cm. The examination confirmed the presence of a highly differentiated squamous cell carcinoma (Figure 1C), staged according to pTNM, American Joint Committee on Cancer 8th Edition: Phase IIIA (T2N1M0). As shown in Table 1, a pathological investigation demonstrated nerve invasion, while immunohistochemistry indicated proficient mismatch repair (pMMR). Subsequently, an adjuvant DP chemotherapy regimen (cisplatin 20 mg through intravenous infusion on days 1-5 combined with doxorubicin 60 mg on days 1 and 8) was initiated (one cycle per 3 wk) from postoperative day 37 to July 29, 2021. The administration of treatment was delayed due to the development of anemia. Consequently, a total of three cycles were carried out during this period. According to the RECIST 1.1 guideline, the condition was evaluated as a stable disease.
| Gene | Mutant/variation | Plasma | Esophagus | Duodenum | Liver |
| CDKN2A | c.322G>T (p.D108Y) | - | 22.0% | - | - |
| NF1 | c.6173 7127-1115del | 9.1% | - | 3.2% | 0.9% |
| TP53 | c.560-2A>T | 0.1% | 15.4% | - | - |
| TP53 | c.557 558delinsG | - | 13.1% | - | - |
| APC | Deletion mutation | 7.8% | - | 8.2% | 1.5% |
| CCND1 | Amplification | - | CN:5.1 | - | - |
| FGF19 | Amplification | - | CN:4.4 | - | - |
| MDM2 | Amplification | - | - | CN:4.4 | - |
| SMAD4 | c.456 478dup | 23.4% | - | 17.1% | 5.9% |
| SMARCA4 | c.2506G>T (p.G836*) | - | - | 6.9% | - |
| CREBBP | c.6666 6677del | 0.1% | - | - | 3.4% |
| EP300 | c.4751 4752delinsAT | - | 12.2% | - | - |
| GATA3 | c.527 528delinsAG | - | - | 2.1% | - |
| RPTOR | c.2000T>C (p.L667P) | - | 2.3% | - | - |
| TMBs | 5.1/Mb (50.4%) | 8.2/Mb (29%) | 4.1/Mb (59.3%) | 3.1/Mb (68.9%) | |
| Microsatellite analysis | MSS/MSI-L | MSS/MSI-L | MSS/MSI-L | MSS/MSI-L | |
| MMR-related mutations | None | None | None | None | |
| Immunohistochemistry | |||||
| CR | - | ||||
| D2-40 | - | ||||
| Ki67 | About 67%+ | About 70%+ | |||
| P40 | + | - | - | ||
| S-100 | + | ||||
| SMA | + | ||||
| CAM5.2 | + | ||||
| CD56 | - | ||||
| CgA | - | ||||
| P63 | - | ||||
| Syn | - | ||||
| CDH17 | - | ||||
| CDX2 | Weak + | ||||
| CK19 | + | ||||
| CK20 | - | ||||
| CK7 | + | ||||
| SATB2 | - | ||||
| TTF | - | ||||
| Villin | + | ||||
| Her2 | + | + | |||
| MLH1 | Complete expression | Complete expression | |||
| MSH2 | Complete expression | Complete expression | |||
| MSH6 | Complete expression | Complete expression | |||
| MSH6 | Complete expression | Complete expression | |||
| PD-L1 | < 1%+ | CPS < 1 | |||
| EBER | - | - | |||
| EGFR | 3+ | 2+ | |||
| VEGF | - | - | |||
| CD15 | Part+ | - | Part- | ||
| CD163 | + | + | + | ||
| CD4 | About 5%+ | About 8%+ | About 10%+ | ||
| CD68 | + | + | + | ||
| CD8 | About 10%+ | About 5%+ | About 15%+ | ||
The patient had a history of hypertension and syphilis with symptomatic treatment, and a 30-year history of smoking. However, he denied alcohol consumption, and did not report any family history of malignant tumors.
On physical examination, the vital signs were as follows: Body temperature, 36.5 °C; blood pressure, 137/88 mmHg; heart rate, 83 beats per min; respiratory rate, 18 breaths per min. Furthermore, the patient exhibited an anemic appearance without iris and skin jaundice. There was no abdominal pressure or percussion pain.
The levels of serum tumor markers were mostly normal (carcinoembryonic antigen, 2.79 ng/mL; carbohydrate antigen 19-9, < 2 U/mL), except for alpha-fetoprotein (17.2 ng/mL; normal range: 0-7 ng/mL) without clinical significance. The concentration of hemoglobin in blood was 68 g/L. There were no abnormalities found in other routine blood, urine, and fecal analyses.
During the postoperative checkup, CT enhancement of the upper abdomen (September 28, 2021) revealed multiple liver metastases (diameter of the largest lesion: 1.5 cm) (Figure 1D). After consultation, the patient was referred to the Department of Oncology for treatment. On October 19, 2021, a needle biopsy of liver mass was performed under ultrasound guidance. The postoperative pathological findings suggested that the liver lesion was compatible with invasive intermediate differentiated adenocarcinoma (Figure 1E). According to the immunohistochemistry findings, the biliopancreatic duct, gastric, and small intestinal sources were considered, as detailed in Table 1. Before the biopsy, a gastroscopy and magnetic resonance enhancement of the abdomen were performed additionally. The former revealed the presence of an ulcerative neoplasm in the descending portion of the duodenum (Figure 1F). The latter indicated multiple metastases in the liver, occupancy of segments 2-3 of the duodenum, and the need for the identification of spinal metastases (Figure 1G). Consequently, a further bone scan was carried out, which detected an abnormal radio concentration lesion in the right iliac bone. Ultimately, pathological examination of the biopsied specimen confirmed a moderately to poorly differentiated DA (Figure 1H), with immunohistochemistry indicating a combined positive score < 1 and pMMR (Table 1). Additionally, the esophagus, duodenum, hepatic lesions, and peripheral blood of the patient were analyzed for 473 genetic loci (Table 1).
Based on the examination results and medical history, the patient was eventually diagnosed with esophageal squamous carcinoma postoperative stage IIIA (pT2N1M0) and DA stage IV (cTxNxM1) (liver metastasis, bone metastasis) after multi-disciplinary evaluation.
The patient received two cycles of XELOX (oxaliplatin 160 mg through intravenous infusion on day 1, combined with capecitabine 1.5 g orally twice daily on days 1-14 every 21 d). This was followed by CT enhancement performed on October 22, 2021, to evaluate progressive disease (PD). Therefore, from December 9, 2021, the treatment plan was changed to GS (gemcitabine 1.2 g through intravenous infusion on days 1 and 8, combined with tegafur 40 mg orally twice daily on days 1-14 every 21 d) along with a programmed death-1 (PD-1) inhibitor (sintilimab 200 mg through intravenous infusion on day 1). However, despite the two cycles of chemotherapy, the condition continued to be rated as PD (January 28, 2022) by CT. Due to the high cost of sintilimab, the regimen was changed to one cycle of monotherapy with irinotecan (200 mg through intravenous infusion on day 1) on January 29, 2022. The patient later declined to continue a second cycle of irinotecan chemotherapy due to a low nutritional state and prolonged grade IV myelosuppression. The tumor continued to grow rapidly after two cycles of immunotherapy with sintilimab again, and all anti-cancer therapy was discontinued.
The patient was eventually followed up until clinical death on June 18, 2022 (Figure 2), with an overall survival (OS) of 16.6 mo.
The prevalence of MPMNs is increasing in parallel with the incidence of malignancies, the continual improvement of diagnostic models, and the extended life of patients with tumors. MPMNs represent 0.7%-11.7% of all cancer cases worldwide[3]. In China, this rate is only 0.99%[4]. A multicenter investigation demonstrated that MPMNs are more commonly detected in individuals aged > 65 years. Men are associated with a higher incidence rate than women, and MC is significantly more common than SC[5]. MPMNs are prevalent in the digestive system[6]. The morbidity rate of MPMNs linked to esophageal cancer ranges from 9.5% to 21.9%[7], with gastric (4.7%), head and neck (2.7%), colorectal (1.2%), and lung (0.7%) cancer being the most common types of combined malignancies. It has also been discovered that approximately one in five patients with ESCC who survive > 6 mo in Western societies develop a second primary cancer within 15 years[8]. Notably, due to the rarity of small bowel tumors and their nonspecific symptoms[9], primary DA and associated MPMNs are rarely reported. Even fewer studies that examine the genomics and immunomics of MPMNs in depth have been published. In this article, we provide a thorough assessment based on the current diagnostic and therapeutic options for MPMNs, taking into account the case of MC. We also conducted an extensive evaluation of the immune microenvironment features of primary and metastatic tumors. Through the analysis of histology data, we sought to: (1) Identify the reasons responsible for the poor prognosis and treatment resistance observed in this case; and (2) Offer novel diagnostic and therapeutic approaches for MPMNs.
The etiology of MPMNs has not been identified; potential causes include abnormal activation of oncogenes, silencing of oncogenes, epigenetic alterations, chromosomal instability, immunodeficiency, environmental exposure, and unhealthy lifestyle habits[10]. The patient in this case had a long history of smoking, which is a risk factor for MPMNs. Of note, the esophagus and duodenum originate in the foregut, and mutation of the lining cells during intrauterine life cannot be excluded in this case.
Tumor recurrence (including metastases) should be distinguished from the second primary for an accurate diagnosis of MPMNs. Firstly, the initial gastroscopic examination at another regional center hospital without pathological biopsies revealed the presence of ulcerative lesions in the duodenal bulb. This finding emphasized the need for a comprehensive assessment at the time of diagnosis of the first tumor. Moreover, a thorough histological analysis of each abnormal lesion is crucial. Secondly, rather than automatically assuming that newly discovered lesions are tumor metastases, clinicians ought to be alert to any new lesions that arise while a patient is receiving therapy. There is a rare possibility of primary or metastatic lesion involvement in the duodenum. Of note, lung cancer, renal cell carcinoma, breast cancer, and malignant melanoma are the most common types of primary tumors that metastasize to the pancreaticoduodenal region[11]. Thirdly, though rare, the incidental detection of one or more additional primary tumors during CT staging of a patient with a known malignancy is possible. Following the detection of masses in the liver by radiologists, an intensive search and identification of the primary site should be performed. The selection between CT, magnetic resonance imaging, positron emission tomography, and ultrasound depends on the tumor type or body region[12]. However, CT (especially contrast-enhanced CT) remains the preferred modality for the staging of tumors and evaluation of treatment efficacy in patients diagnosed with cancer[13]. In addition, the appearance and progression of liver metastases on CT were accompanied by an increase in the levels of carbohydrate antigen 72-4 and carcinoembryonic antigen (Table 2). A dramatic increase in the levels of carcinoembryonic antigen when liver metastases continue to spread and the burden of systemic tumors continues to rise, which may indicate rapid progression of disease.
| Date | Reference range | February 19, 20211 | March 31, 20212 | October 8, 20213 | December 7, 20214 | January28, 20225 | May 14, 20226 |
| CA72-4 (u/mL) | 0-6.9 | 1.64 | 19.1 | 6.48 | 8.61 | 74.6 | 227 |
| CEA (ng/mL) | 0-5 | 2.54 | 2.29 | 2.79 | 14.6 | 47.1 | 742 |
| AFP (ng/mL) | 0-5 | 16.6 | 18.8 | 17.2 | 18.3 | 16.2 | 10.8 |
| NLR | - | 7.25 | 1.47 | 1.76 | 2.21 | 2.21 | 15.89 |
| LMR | - | 1.29 | 4.07 | 3.34 | 2.37 | 2.37 | 1.3 |
| PLR | - | 376.72 | 220.86 | 305.44 | 278.89 | 278.89 | 258.46 |
| IL-6 (pg/mL) | 0-5.3 | - | - | 7.18 | 7.12 | 27.82 | 918.02 |
| IL-8 (pg/mL) | 0-20.6 | - | - | 9.55 | 10.21 | 17.73 | 230.94 |
| ω-3-C22:5 (μmol/L) | 0.74-3.11 | - | - | 0.448 | 0.529 | 0.348 | 0.295 |
| ω-6-C22:5 (μmol/L) | 0.37-1.86 | - | - | 0.293 | 0.193 | 0.138 | 0.136 |
| ω-6/ω-3 | < 10 | - | - | 13.68 | 18.75 | 27.01 | 8.04 |
| UDCA (nmol/L) | 40-758 | - | - | 4.6 | 4.7 | 11.3 | 13.9 |
Factors that affect the prognosis of patients with MPMNs include age at initial cancer diagnosis (≥ 60 years) and tumor stage[14]. The 2- and 5-year survival rates of patients with MPMNs are 40.8% and 4.6%, respectively[4]. The median OS for patients with MC-MPMNs and SC-MPMNs is 91 mo and 30 mo, respectively[15]. The presence of MC-MPMNs and patient age < 60 years at the time of initial diagnosis of the primary tumor indicate a good prognosis. Nevertheless, the OS of the present patient was only 16.6 mo. Therefore, it is necessary to further analyze the reasons responsible for the poor prognosis. Although studies on the tumor microenvironment (TME) have yielded some promising results, there is a lack of investigations focusing on MPMNs. In this case, we examined several areas (i.e., genomics, immunomics, inflammatory markers, and lipid metabolism) to accurately explain the histological features of the three malignancies identified in this patient.
Firstly, the development of second malignancies is largely caused by genetic susceptibility, with approximately 100 mutated genes causing one or more cancers[16]. The “multicentric origin” theory[17,18] suggests that different primary cancers in the same patient may have different mutation profiles and be driven by different genes. Patients with two or more characteristic cancers (synchronous or asynchronous) should undergo genetic testing. Therefore, in this case, the patient underwent prompt genetic testing after the discovery of DA. Table 1 demonstrates the results of gene high-throughput sequencing. Interestingly, cyclin-dependent kinase inhibitor 2A (CDKN2A), tumor protein p53 (TP53), cyclin D1 (CCND1), and E1A binding protein p300 (EP300) showed mutations only in ESCC tissue, while neurofibromin 1, adenomatosis polyposis coli (APC), and SMAD family member 4 (SMAD4) showed mutations in peripheral blood, the duodenal tumor, and liver metastatic carcinoma. Similarly, genes involved in the cell cycle and apoptosis regulation (e.g., CDKN2A, TP53, and CCND1) are mutated in 99% of ESCC cases[19]. In particular, increased CDKN2A gene deletion in somatic cells, which is mainly reported in lung and upper gastrointestinal tumors, may provide an early warning sign of esophageal cancer. In this case, the rate of CDKN2A gene mutation in the esophageal tumor tissue was 22%, suggesting a poor prognosis. In addition, the EP300 gene is involved in the epigenetic process of histone modification in ESCC, and is associated with poor prognosis[20,21]. We found few genetic studies on primary duodenal cancer. The detection of duodenal lesions revealed in this case was based on the findings of Schrock et al[22] in genomic studies of small bowel cancer. The investigators of that study concluded that APC and SMAD4 are commonly altered genes, and the rate of APC mutations is relatively low.
Secondly, some immunohistological features have been identified as susceptibility factors for second primary carcinogenesis. It has been suggested that microsatellite instability (MSI) and defective DNA damage repair are associated with the occurrence of MPMNs[23]. The probable mechanism underlying this relationship is the existence of Lynch syndrome, a genetic disorder caused by mutations in mismatch repair genes. MSI appears to be more prevalent in MPMNs than sporadic cancers. Cancer of the small intestine belongs to the Lynch syndrome spectrum of tumors. The lifetime risk in carriers is 4%, independent of the development of colon cancer[24]. Immunohistochemical typing of both primary tumor tissues in this case revealed pMMR. In addition, gene sequencing suggested that the MSI status was microsatellite stability, indicating that this patient was less likely to have Lynch syndrome and suggesting possible low responsiveness to immunotherapy.
Thirdly, the TME is a complex system consisting of multiple cell types. Previous studies showed that CD68 and CD163 are phenotypic markers of M1- and M2-type tumor-associated macrophages (TAMs), respectively[25]. It has been shown that increased numbers of CD163 + M2 macrophages contribute to angiogenesis, tumor aggressiveness, and ESCC progression[26]. These processes can deplete CD8+ T cells that exert specific anti-tumor effects via the PD-1/programmed death-ligand 1 (PD-1/PD-L1) pathway, thereby increasing the risk of immune escape of tumor cells[27]. However, there is controversy regarding the relationship between CD68 + M1 macrophages and the prognosis of gastrointestinal malignancies. Wang et al[28] concluded that the extent of CD68+ macrophage infiltration was negatively associated with survival time and prognosis. In contrast, Tang et al[29] argued that the abundance of CD68+ TAMs is not associated with ESCC progression, while that of CD163+ M2 TAMs is a potential risk factor. Based on data reported by previous studies and the validation of the clinical prognosis prediction of this patient, we performed immunohistochemical staining for CD68 and CD163 molecules in three cancerous tissues. The results showed consistently positive expression; high expression of CD68 and CD163 was associated with a poorer prognosis in this patient[30]. In addition to TAMs, tumor-associated neutrophils may promote T cell-mediated immunity through costimulatory molecules that enhance the proliferation of CD4+ and CD8+ T cells and increase the anti-tumor activity in early-stage disease[31]. As a cell surface glycoprotein regulated by neutrophil function, CD15 is thought to be associated with adverse OS[32]. In this case, the esophageal cancer tissue exhibited positivity for CD15, and the patient had a poor prognosis. These findings are consistent with those of previous studies. Therefore, genomics and immunomics play an important role in determining the degree of tumor malignancy, and can further guide the assessment of the prognosis of MPMNs.
Fourthly, immune-inflammatory cells in peripheral blood play an important role in tumors and can be used to predict prognosis and assess outcomes. It has been reported that the neutrophil-lymphocyte ratio (NLR), lymphocyte-monocyte ratio (LMR), and platelet-lymphocyte ratio (PLR) are useful in predicting the prognosis of ESCC. For instance, preoperative high NLR (> 3.29) and low LMR (< 2.95) in patients with ESCC are associated with worse OS[33,34]. Such evidence reflects an imbalance between the pro-cancer inflammatory response and the anti-cancer immune response. Moreover, LMR has been previously proposed as a poor prognostic factor for DA[35]. Furthermore, high PLR is associated with poor OS/cancer-specific survival, event-free survival, and malignant phenotype in tumors such as ESCC[36]. In this case, although the preoperative NLR was only 2.09, the LMR was at a low level during radical treatment of esophageal cancer (Table 2), and the PLR was at a high level. These findings are consistent with the poor prognosis of this patient. The overall surveillance trend showed a progressive increase in NLR accompanied by the development of DA and the development of metastases; the opposite was true for LMR. These observations suggest that lower lymphocyte counts and relatively weak anti-tumor immunity may contribute to increased tumor size and poor prognosis. Additionally, the circulating blood inflammation-associated cytokine interleukin-6 (IL-6) is considered a typical pro-tumor cytokine in the IL-6 cytokine family. It is involved in the formation of the local TME and is considered a hallmark feature of tumor growth initiation and progression[37,38]. IL-8 is a pro-inflammatory chemokine, and/or its receptors are expressed in cancer cells, endothelial cells, and TAMs. Increased expression of IL-8 is associated with tumor angiogenesis, tumorigenicity, and metastasis[39]. The present patient had high IL-6 levels (7.18 pg/mL; normal range: 0-5.3 pg/mL) at the time of diagnosis of DA, showing a progressive increase with tumor progression. Following the discontinuation of chemotherapy, the levels of IL-6 and IL-8 rose rapidly at 918.02 pg/mL and 230.94 pg/mL, respectively. These data were highly suggestive of rapid disease progression.
Finally, reprogramming of lipid metabolism is one of the most prominent metabolic alterations in cancer, including fatty acid and bile acid (BA). It has been suggested that ω-3 polyunsaturated fatty acids (PUFA) may exert an anti-angiogenesis effect in tumors, inhibit cancer cell invasion and metastasis, and reverse chemotherapy multi-drug resistance in tumor cells. In contrast, ω-6 PUFA and total PUFA may exacerbate the risk of cancer[40]. According to the fatty acid metabolism indices of this patient (Table 2), the levels of ω-3 PUFA were low, while those of ω-6/ω-3 were higher than the maximum normal value of 10. The high levels of eicosatetraenoic acid, which belongs to the ω-6 group, led to the analysis of the predominance of cancer-promoting factors in this case. Predominance of pro-carcinogenic factors was suggested. In addition, the high-BA environment could promote apoptosis and inhibit the migration of cancer cells, particularly in colon cancer cells[41,42]. However, BA is metabolized by the intestinal microbiota; disruption of the balance between the two systems can lead to abnormal BA concentrations and pools, triggering the abnormal proliferation of intestinal stem cells[41]. In particular, it is thought that ursodeoxycholic acid enhances anti-tumor immunity by degrading transforming growth factor-β, thereby inhibiting the differentiation and activation of regulatory T cells. Moreover, it synergizes with PD-L1 to enhance tumor-specific immune memory[43]. Consequently, the levels of ursodeoxycholic acid in this patient were low throughout the evaluation. This observation is associated, to some extent, with the continuous progression of DA and poor efficacy of immunotherapy.
Currently, there are no standard guidelines or expert consensus for the comprehensive treatment of MPMNs. Therapy is generally based on a combination of several factors, such as patient age, clinicopathological features of the different tumors, biological and genomic expression profiles, life expectancy, and comorbid diseases. For MC-MPMNs, the treatment approach invariably involves sequential treatment of all tumors; however, for SC-MPMNs, individualized and unique treatment plans are generally developed after multidisciplinary discussions[44]. In this case, the patient presented with two successive asynchronous tumors of different histological origins, namely esophageal squamous epithelial carcinoma and DA. Therefore, radical surgery combined with adjuvant chemotherapy was performed for the esophageal tumor, and complete remission was achieved. For the DA and liver metastases, oxaliplatin-based regimens appear to be the most commonly used and effective options in first-line treatment[45]. In this case, the preferred XELOX regimen did not prevent PD after two cycles of chemotherapy. This unsatisfactory outcome may be attributed to the development of adverse effects linked to chemotherapy for ESCC, such as malnutrition, and bone marrow suppression. These effects are poorly tolerated by patients with a poor physical status. The combination of metastases suggests that the tumor has progressed to an advanced stage and the general treatment is less effective. Mouse double minute 2 (MDM2) in genomics may reduce the efficacy of chemotherapeutic agents, such as platinum, by inhibiting the action of TP53. It has also been suggested that 5-fluorouracil and capecitabine exhibit poorer efficacy in patients with TP53 mutation vs wild-type TP53[46].
The role and efficacy of emerging immunotherapies in DA are currently under investigation. The investigators of the phase II KEYNOTE-158 study concluded that pembrolizumab is an effective option for previously treated patients with MSI-high small bowel adenocarcinoma[47]. Given the possible benefit of immunosuppression with negative PD-L1 expression, this patient was treated with sintilimab in combination with second-line therapy.
However, the effectiveness of immunotherapy is limited, probably due to the following reasons. Firstly, the patient was in an immunosuppressed state before immunotherapy: The CD4+ and CD8+ T cells in three tumor tissues were poorly infiltrated, and immune cells (e.g., lymphocytes, B cells, and natural killer cells) in peripheral blood were below the normal range, particularly neutrophils, CD4+ T and CD8+ T cells. Therefore, overcoming the immunosuppressed state is a major challenge for immunotherapy. Secondly, the PD-L1 expression in the second primary cancer was negative. As an immune checkpoint inhibitor (ICI), sintilimab cannot block the immune checkpoint pathway or reactivate T cell-mediated anti-tumor immunity. Thirdly, the low tumor mutational burden in all three pathological tissues indicates that neoantigens are not exposed to the immune system, thus affecting ICI therapy. Fourthly, both primary carcinomas and metastases are in a microsatellite stable state/pMMR. The immune escape mechanisms in these tumors include the expression of relatively low levels of immunosuppressive ligands, low tumor mutational load, and lack of immune cell infiltration, compromising the effectiveness of immunotherapy. Fifthly, the mutational status of TP53 (an important oncogene in humans) correlated with the efficacy of ICIs. Sixthly, MDM2 gene amplification in liver metastatic tumor tissues and immunohistochemical analysis of the duodenal pathology suggested the involvement of epidermal growth factor receptor (2+), which is associated with hyper-progression during immunotherapy[48]. Finally, peripheral blood findings indicated the presence of Epstein-Barr virus, which is thought to transform tumor precursor cells into Epstein-Barr virus-associated malignancies, and can shape the immunosuppressive microenvironment to induce oncogenesis[49,50]. It is proposed that the poor efficacy and poor prognosis observed in this patient are the results of multiple factors and omics-coordinated regulation.
In this article, we report the case of a middle-aged male with MC-MPMNs, diagnosed with ESCC and DA with liver and bone metastases after an 8.4-mo interval. Based on clinical and pathological features, chemotherapy and immunotherapy were administered against the second primary tumor after multidisciplinary treatment. The patient had an OS of 16.6 mo. Such cases raise awareness among clinicians regarding MPMNs. Although the incidence of MPMNs is low, regular follow-up, vigilance, and comprehensive analysis are crucial for the diagnosis of second primary malignancies. Moreover, in addition to tumor markers, endoscopy, and imaging techniques, emerging inflammatory immunomarkers, genomics, immunomics, and metabolomics can reveal the high heterogeneity of tumors. This approach may facilitate the selection of treatment, improve efficacy, and predict prognosis. Due to the rarity of MPMNs, enhanced collaboration among multiple clinical centers is warranted to conduct prospective clinical studies. Such studies would require expanded sample sizes for TME and multi-omics studies concerning MPMNs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Caboclo JLF, Brazil; Corvino A, Italy; Losurdo G, Italy; Gupta V, India; Dilek ON, Turkey; Kalayarasan R, India S-Editor: Wang JJ L-Editor: A P-Editor: Yuan YY
| 1. | Warren S. Multiple primary malignant tumors: a survey of the literature and statistical study. Am J Cancer. 1932;16:1358-1414. [Cited in This Article: ] |
| 2. | Moertel CG, Dockerty MB, Baggenstoss AH. Multiple primary malignant neoplasms. II. Tumors of different tissues or organs. Cancer. 1961;14:231-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 3. | Tripodi D, Cannistra' C, Gagliardi F, Casella G, Lauro A, De Luca A, Amabile MI, Palumbo P, Pironi D, Mascagni D, D'Andrea V, Vergine M, Sorrenti S. Coincidental or Causal? Concurrence of Colorectal Carcinoma with Primary Breast Cancer. Dig Dis Sci. 2022;67:437-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Liu Z, Liu C, Guo W, Li S, Bai O. Clinical analysis of 152 cases of multiple primary malignant tumors in 15,398 patients with malignant tumors. PLoS One. 2015;10:e0125754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Feller A, Matthes KL, Bordoni A, Bouchardy C, Bulliard JL, Herrmann C, Konzelmann I, Maspoli M, Mousavi M, Rohrmann S, Staehelin K, Arndt V; NICER Working Group. The relative risk of second primary cancers in Switzerland: a population-based retrospective cohort study. BMC Cancer. 2020;20:51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Si L, Feng Y, Wang Y, Zhong J, Sun Z, Li X, Sun Y. Clinical and pathological characteristics of multiple primary malignant neoplasms cases. Int J Clin Pract. 2021;75:e14663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | van de Ven SEM, Falger JM, Verhoeven RHA, Baatenburg de Jong RJ, Spaander MCW, Bruno MJ, Koch AD. Increased risk of second primary tumours in patients with oesophageal squamous cell carcinoma: a nationwide study in a Western population. United European Gastroenterol J. 2021;9:497-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Muto M, Takahashi M, Ohtsu A, Ebihara S, Yoshida S, Esumi H. Risk of multiple squamous cell carcinomas both in the esophagus and the head and neck region. Carcinogenesis. 2005;26:1008-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 86] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 9. | Campanile F, Maurea S, Mainenti P, Corvino A, Imbriaco M. Duodenal involvement by breast cancer. Breast J. 2012;18:615-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Buchbjerg T, Fristrup C, Mortensen MB. The incidence and prognosis of true duodenal carcinomas. Surg Oncol. 2015;24:110-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Medina-Franco H, Halpern NB, Aldrete JS. Pancreaticoduodenectomy for metastatic tumors to the periampullary region. J Gastrointest Surg. 1999;3:119-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Corvino A, Setola SV, Sandomenico F, Corvino F, Catalano O. Synchronous tumours detected during cancer patient staging: prevalence and patterns of occurrence in multidetector computed tomography. Pol J Radiol. 2020;85:e261-e270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Corvino A, Corvino F, Radice L, Catalano O. Synchronous mucinous colonic adenocarcinoma and multiple small intestinal adenocarcinomas: report of a case and review of literature. Clin Imaging. 2015;39:538-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Wang H, Hou J, Zhang G, Zhang M, Li P, Yan X, Ma Z. Clinical characteristics and prognostic analysis of multiple primary malignant neoplasms in patients with lung cancer. Cancer Gene Ther. 2019;26:419-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Etiz D, Metcalfe E, Akcay M. Multiple primary malignant neoplasms: A 10-year experience at a single institution from Turkey. J Cancer Res Ther. 2017;13:16-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Cybulski C, Nazarali S, Narod SA. Multiple primary cancers as a guide to heritability. Int J Cancer. 2014;135:1756-1763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Peng L, Zeng Z, Teng X, Chen Z, Lin L, Bao H, Shao YW, Wang Y, Dong Y, Zhao Q. Genomic profiling of synchronous triple primary tumors of the lung, thyroid and kidney in a young female patient: A case report. Oncol Lett. 2018;16:6089-6094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Kang GH, Kim CJ, Kim WH, Kang YK, Kim HO, Kim YI. Genetic evidence for the multicentric origin of synchronous multiple gastric carcinoma. Lab Invest. 1997;76:407-417. [PubMed] [Cited in This Article: ] |
| 19. | Gao YB, Chen ZL, Li JG, Hu XD, Shi XJ, Sun ZM, Zhang F, Zhao ZR, Li ZT, Liu ZY, Zhao YD, Sun J, Zhou CC, Yao R, Wang SY, Wang P, Sun N, Zhang BH, Dong JS, Yu Y, Luo M, Feng XL, Shi SS, Zhou F, Tan FW, Qiu B, Li N, Shao K, Zhang LJ, Xue Q, Gao SG, He J. Genetic landscape of esophageal squamous cell carcinoma. Nat Genet. 2014;46:1097-1102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 512] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 20. | Bi Y, Kong P, Zhang L, Cui H, Xu X, Chang F, Yan T, Li J, Cheng C, Song B, Niu X, Liu X, Xu E, Hu X, Qian Y, Wang F, Li H, Ma Y, Yang J, Liu Y, Zhai Y, Wang Y, Zhang Y, Liu H, Liu J, Wang J, Cui Y, Cheng X. EP300 as an oncogene correlates with poor prognosis in esophageal squamous carcinoma. J Cancer. 2019;10:5413-5426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Song Y, Li L, Ou Y, Gao Z, Li E, Li X, Zhang W, Wang J, Xu L, Zhou Y, Ma X, Liu L, Zhao Z, Huang X, Fan J, Dong L, Chen G, Ma L, Yang J, Chen L, He M, Li M, Zhuang X, Huang K, Qiu K, Yin G, Guo G, Feng Q, Chen P, Wu Z, Wu J, Zhao J, Luo L, Fu M, Xu B, Chen B, Li Y, Tong T, Wang M, Liu Z, Lin D, Zhang X, Yang H, Zhan Q. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 721] [Cited by in F6Publishing: 786] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 22. | Schrock AB, Devoe CE, McWilliams R, Sun J, Aparicio T, Stephens PJ, Ross JS, Wilson R, Miller VA, Ali SM, Overman MJ. Genomic Profiling of Small-Bowel Adenocarcinoma. JAMA Oncol. 2017;3:1546-1553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 23. | Cercato MC, Colella E, Ferraresi V, Diodoro MG, Tonachella R. Report of two cases of quintuple primary malignancies and review of the literature. Anticancer Res. 2008;28:2953-2958. [PubMed] [Cited in This Article: ] |
| 24. | ten Kate GL, Kleibeuker JH, Nagengast FM, Craanen M, Cats A, Menko FH, Vasen HF. Is surveillance of the small bowel indicated for Lynch syndrome families? Gut. 2007;56:1198-1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Hu JM, Liu K, Liu JH, Jiang XL, Wang XL, Chen YZ, Li SG, Zou H, Pang LJ, Liu CX, Cui XB, Yang L, Zhao J, Shen XH, Jiang JF, Liang WH, Yuan XL, Li F. CD163 as a marker of M2 macrophage, contribute to predicte aggressiveness and prognosis of Kazakh esophageal squamous cell carcinoma. Oncotarget. 2017;8:21526-21538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 26. | Sugimura K, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y. High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111:752-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Yang H, Zhang Q, Xu M, Wang L, Chen X, Feng Y, Li Y, Zhang X, Cui W, Jia X. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol Cancer. 2020;19:41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 196] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 28. | Wang XL, Liu K, Liu JH, Jiang XL, Qi LW, Xie YF, Li JF, Yang L, Chen YZ, Liu CX, Li SG, Cui XB, Zou H, Pang LJ, Zhao J, Qi Y, Cao YW, Liang WH, Jiang JF, Shen XH, Yuan XL, Hu JM, Li F. High infiltration of CD68-tumor associated macrophages, predict poor prognosis in Kazakh esophageal cancer patients. Int J Clin Exp Pathol. 2017;10:10282-10292. [PubMed] [Cited in This Article: ] |
| 29. | Tang Y, Liu JH, Shi ZX, Li Z, Liu HT, Lu P. MicroRNA-133b suppresses cell proliferation and invasion of esophageal squamous cell carcinoma via downregulating TAGLN2 expression. Zhonghua Zhong Liu Za Zhi. 2019;41:91-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 30. | Minami K, Hiwatashi K, Ueno S, Sakoda M, Iino S, Okumura H, Hashiguchi M, Kawasaki Y, Kurahara H, Mataki Y, Maemura K, Shinchi H, Natsugoe S. Prognostic significance of CD68, CD163 and Folate receptor-β positive macrophages in hepatocellular carcinoma. Exp Ther Med. 2018;15:4465-4476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A, Litzky L, Hancock WW, Conejo-Garcia JR, Feldman M, Albelda SM, Singhal S. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124:5466-5480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 443] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 32. | Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e98259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 33. | Li B, Xiong F, Yi S, Wang S. Prognostic and Clinicopathologic Significance of Neutrophil-to-Lymphocyte Ratio in Esophageal Cancer: An Update Meta-Analysis. Technol Cancer Res Treat. 2022;21:15330338211070140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Lv X, Han S, Xu B, Deng Y, Feng Y. The value of complete blood count for the prognosis analysis of preoperative esophageal squamous cell carcinoma. BMC Cancer. 2021;21:1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Shi J, Liu S, Cao J, Shan S, Zhang J, Wang Y. Development and validation of lymph node ratio-based nomograms for primary duodenal adenocarcinoma after surgery. Front Oncol. 2022;12:962381. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 36. | Sun Y, Zhang L. The clinical use of pretreatment NLR, PLR, and LMR in patients with esophageal squamous cell carcinoma: evidence from a meta-analysis. Cancer Manag Res. 2018;10:6167-6179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 406] [Cited by in F6Publishing: 435] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 38. | Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 475] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 39. | Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735-6741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1408] [Cited by in F6Publishing: 1522] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 40. | Hanson S, Thorpe G, Winstanley L, Abdelhamid AS, Hooper L; PUFAH group. Omega-3, omega-6 and total dietary polyunsaturated fat on cancer incidence: systematic review and meta-analysis of randomised trials. Br J Cancer. 2020;122:1260-1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 41. | Wang S, Dong W, Liu L, Xu M, Wang Y, Liu T, Zhang Y, Wang B, Cao H. Interplay between bile acids and the gut microbiota promotes intestinal carcinogenesis. Mol Carcinog. 2019;58:1155-1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 42. | Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1054] [Cited by in F6Publishing: 984] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 43. | Shen Y, Lu C, Song Z, Qiao C, Wang J, Chen J, Zhang C, Zeng X, Ma Z, Chen T, Li X, Lin A, Guo J, Cai Z. Ursodeoxycholic acid reduces antitumor immunosuppression by inducing CHIP-mediated TGF-β degradation. Nat Commun. 2022;13:3419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 44. | Ágoston EI, Somorácz Á, Madaras L, Zaránd A, Szentmártoni G, Orosz Z, Dank M, Baranyai Z. Successful treatment of three synchronous primary malignant tumours-reflection on surgical, pathological and oncological aspects and decision making. J Surg Case Rep. 2018;2018:rjy041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Overman MJ, Varadhachary GR, Kopetz S, Adinin R, Lin E, Morris JS, Eng C, Abbruzzese JL, Wolff RA. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27:2598-2603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 46. | Sabeti Aghabozorgi A, Moradi Sarabi M, Jafarzadeh-Esfehani R, Koochakkhani S, Hassanzadeh M, Kavousipour S, Eftekhar E. Molecular determinants of response to 5-fluorouracil-based chemotherapy in colorectal cancer: The undisputable role of micro-ribonucleic acids. World J Gastrointest Oncol. 2020;12:942-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 47. | Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 942] [Cited by in F6Publishing: 1574] [Article Influence: 314.8] [Reference Citation Analysis (0)] |
| 48. | Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res. 2017;23:4242-4250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 538] [Cited by in F6Publishing: 649] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 49. | de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1583] [Cited by in F6Publishing: 1710] [Article Influence: 142.5] [Reference Citation Analysis (0)] |
| 50. | Tan GW, Visser L, Tan LP, van den Berg A, Diepstra A. The Microenvironment in Epstein-Barr Virus-Associated Malignancies. Pathogens. 2018;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |