Published online Nov 27, 2022. doi: 10.4240/wjgs.v14.i11.1250
Peer-review started: August 2, 2022
First decision: September 4, 2022
Revised: September 27, 2022
Accepted: November 4, 2022
Article in press: November 4, 2022
Published online: November 27, 2022
Anastomotic leakage (AL) is a fatal complication in patients with rectal cancer after undergoing anterior resection. However, the role of abdominal composition in the development of AL has not been studied.
To investigate the relationship between abdominal composition and AL in rectal cancer patients after undergoing anterior resection.
A retrospective case-matched cohort study was conducted. Complete data for 78 patients with AL were acquired and this cohort was defined as the AL group. The controls were matched for the same sex and body mass index (± 1 kg/m2). Parameters related to abdominal composition including visceral fat area (VFA), subcutaneous fat area (SFA), subcutaneous fat thickness (SFT), skeletal muscle area (SMA), skeletal muscle index (SMI), abdominal circumference (AC), anterior to posterior diameter of abdominal cavity (APD), and transverse diameter of abdominal cavity (TD) were evaluated based on computed tomography (CT) images using the following Hounsfield Unit (HU) thresholds: SFA: -190 to -30, SMA: -29 to 150, and VFA: -150 to -20. The significance of abdominal composition-related parameters was quantified using feature importance analysis; an artificial intelligence method was used to evaluate the contribution of each included variable.
Two thousand two hundred and thirty-eight rectal cancer patients who underwent anterior resection from 2010 to 2020 in a large academic hospital were investigated. Finally, 156 cases were enrolled in the study. Patients in the AL group showed longer operative time (225.03 ± 55.29 vs 207.17 ± 40.80, P = 0.023), lower levels of preoperative hemoglobin (123.32 ± 21.17 vs 132.60 ±1 6.31, P = 0.003) and albumin (38.34 ± 4.01 vs 40.52 ± 3.97, P = 0.001), larger tumor size (4.07 ± 1.36 vs 2.76 ± 1.28, P < 0.001), and later cancer stage (P < 0.001) compared to the controls. Patients who developed AL exhibited a larger VFA (125.68 ± 73.59 vs 97.03 ± 57.66, P = 0.008) and a smaller APD (77.30 ± 23.23 vs 92.09 ± 26.40, P < 0.001) and TD (22.90 ± 2.23 vs 24.21 ± 2.90, P = 0.002) compared to their matched controls. Feature importance analysis revealed that TD, APD, and VFA were the three most important abdominal composition-related features.
AL patients have a higher visceral fat content and a narrower abdominal structure compared to matched controls.
Core Tip: We investigated the association between abdominal composition and anastomotic leakage in rectal cancer patients who underwent anterior resection in a large academic hospital from 2010 to 2020. The data revealed that patients who developed anastomotic leakage had a higher visceral fat content and a narrower abdominal structure, despite body mass index matching.
- Citation: Shao SL, Li YK, Qin JC, Liu L. Comprehensive abdominal composition evaluation of rectal cancer patients with anastomotic leakage compared with body mass index-matched controls. World J Gastrointest Surg 2022; 14(11): 1250-1259
- URL: https://www.wjgnet.com/1948-9366/full/v14/i11/1250.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i11.1250
Compelling evidence demonstrates that total mesorectal resection (TME) successfully reduces the local recurrence rate of rectal cancer and is the gold standard for managing mid- and low-lying rectal cancer[1-3]. However, the morbidity of anastomotic leakage (AL), a worrisome complication of TME, is on the rise[4]. Once AL develops, it often requires reintervention and can lead to perioperative death and adverse oncology outcomes[5-7]. Early identification of patients at high risk of AL is critical to AL prevention and reduction of the reoperation rate, and will guide intraoperative decisions (for instance on whether to choose a diverting ileostomy or not) and improve perioperative management.
Numerous studies have explored the risk factors associated with AL in rectal cancer patients who underwent anterior resection[8,9]. However, there is no effective approach for predicting AL, implying that potential predictors should be identified. Recent studies show that some abdominal composition related factors are key contributors to AL in patients with colorectal cancer after undergoing surgery[10]. Theoretically, a less visceral fat content and a bigger abdominal volume are more favorable for surgeons to perform anterior resection procedure and thus leads to less technical difficulty, shorter operation time, and lower probability of AL[11]. Computed tomography (CT) images have been employed to assess the possible effects of abdominal composition related parameters, including visceral fat area (VFA) and skeletal muscle index (SMI), on patient surgical outcome[10,12-15]. Large VFA, for instance, is potentially effective in predicting AL in patients with colorectal cancer who received anterior resection despite reports to the contrary[9]. Additionally, SMI, measured by a CT scan of the lower margin of the third lumbar spine, is a reliable indicator of the systemic nutritional status and is associated with perioperative complications[16]. Additional indicators, including abdominal circumference (AC), anterior to posterior diameter of abdominal cavity (APD), and transverse diameter of abdominal cavity (TD), are suggested to exert potential effects on perioperative complications but their roles in AL is unknown.
Considering the impact of abdominal composition on the surgeons and patients, it was hypothesized that the abdominal composition of rectal cancer patients who developed AL after anterior resection may be different from that of individuals with similar body mass index (BMI) who did not develop AL. Here, we compared the abdominal composition between AL patients and sex- and BMI-matched controls.
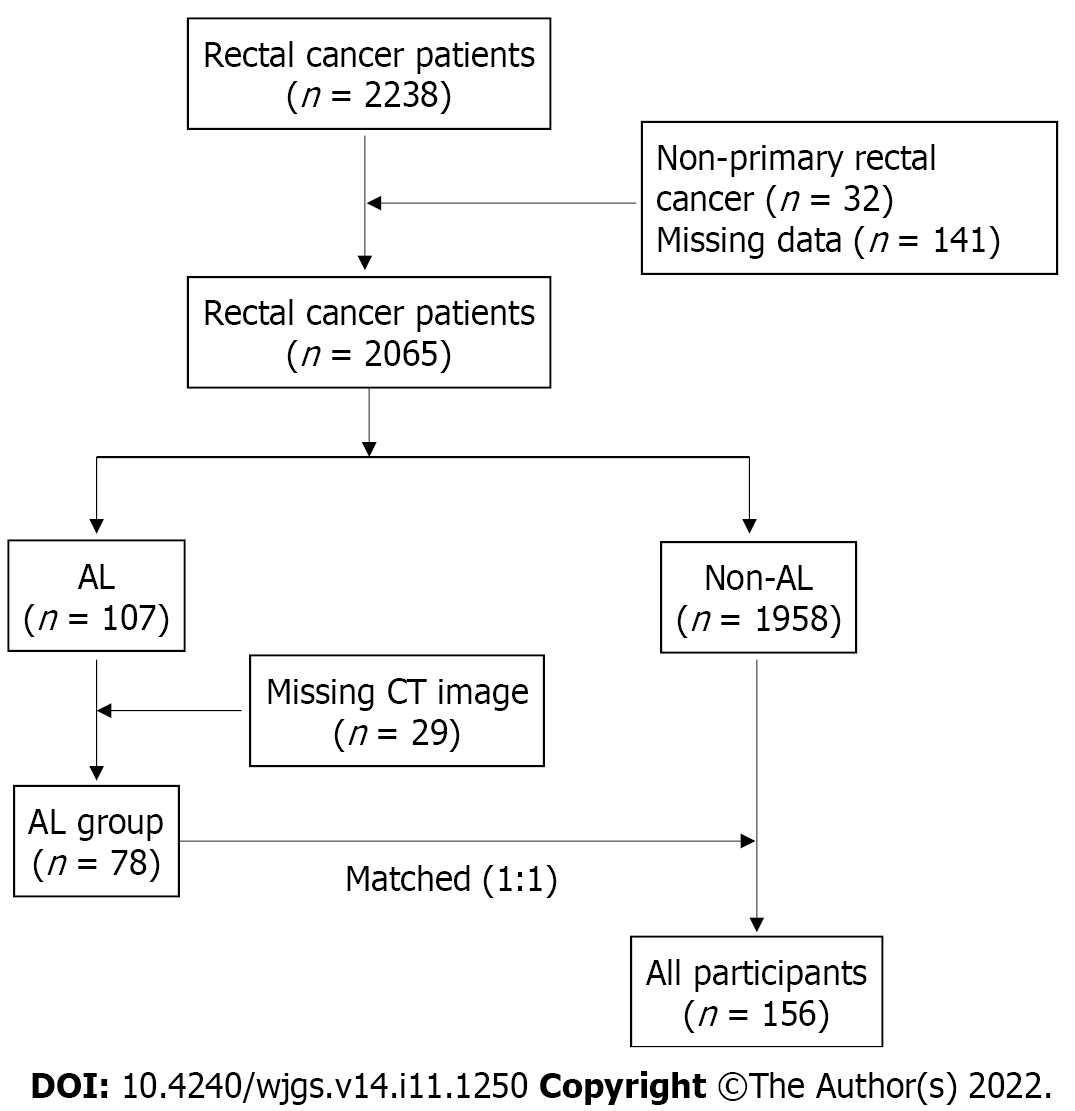
A total of 2238 medical records of rectal cancer patients who underwent anterior resection at our center from January 1, 2010 to January 1, 2020 were reviewed. Of note, 173 patients were excluded due to non-primary rectal adenocarcinoma (n = 32) and missing clinical data (n = 141). All patients underwent a 90-d follow-up. Of the 2065 subjects, 107 (5.18%) developed clinical AL (i.e., grades B and C). Among the AL patients, 29 were excluded for missing CT images, and the remaining 78 were included in the final analysis and defined as the AL group. The control group was matched 1:1 for the same sex and BMI (± 1 kg/m2) from patients who did not develop AL. A flowchart of this study is shown in Figure 1.
In this study, rectal cancer was defined as a tumor located between the dentate line and sacral promontory. AL refers to clinical AL, including grade B and grade C, defined as disruption and defect in intestinal wall integrity at the anastomosis site, making the internal and external compartments communicate with each other[17]. AL diagnosis is contingent on the fecal fluid from pelvic draining or water-soluble contrast agent enema and extra-rectal imaging. Alternatively, when AL was suspected, perianastomotic abscess or effusion detected by CT was examined to diagnose AL. Because water-soluble contrast agent enema is not performed routinely at our center, AL of grade A was not included. The clinical variables gender, age, height, weight, BMI, ASA score, previous abdominal history, hypertension, diabetes, cigarette smoking, alcohol use, tumorous obstruction, preoperative cleansing enema, preoperative antibiotic use, distance between tumor and anal margin, neoadjuvant, preoperative hemoglobin, preoperative albumin, type of operation, tumor size, clinical tumor stage, operation time, number of linear stapler firings, indwelling pelvic drainage tube, indwelling trans-anal tube, and stoma were also considered. Abdominal composition-related parameters assessed included BMI, AC, subcutaneous fat area (SFA), subcutaneous fat thickness (SFT), skeletal muscle area (SMA), SMI, VFA, APD, and TD.
Data of BMI and AC were acquired from medical records, whereas other indicators were examined at the lower margin of the third lumbar (L3) plane of the unenhanced CT image using Slice-O-Matic software (version 5.0; Tomovision, Montreal, Canada). CT images were saved in DICOM (Medical Digital Imaging and Communication) format and retrieved from the institutional database. SFA, SMA, and VFA were measured by setting Hounsfield Unit (HU) thresholds (SFA: -190 to -30, SMA: -29 to 150 and VFA: -150 to -20)[18]. SFT refers to the vertical distance from the linear alba to the skin. SMI was calculated as SMA/hight2 (cm2/m2)[19,20]. APD refers to the vertical distance from the linear alba to the anterior edge of the L3 spine. TD refers to the transverse diameter of the abdominal cavity through the anterior edge of the L3 spine.
Continuous variables are presented as the mean and standard deviation (SD), whereas categorical variables are presented as numerical values (percentages). Student’s t-test and chi-square test were used to compare continuous variables and categorical variables, respectively. A P value of < 0.05 denoted statistical significance. All statistical analyses were performed using IBM SPSS 24.0 (SPSS for Windows, IBM Corporation, Armonk, NY, United States).
Feature importance analysis is an artificial intelligence method used for examining the importance of each included feature. This approach is based on some ensemble learning algorithms, such as random forest and XGboost. In this study, we used the random forest analysis to calculate the importance of each abdominal composition related parameter. Random forest is an ensemble classifier based on a combination of multiple decision trees which are generated through sampling from the original data set and the final predictions are voted by integrating all the trees. Mean decrease accuracy was calculated by randomly permuting a variable to reassess the predictions. If a variable is important, the mean decrease accuracy will show a large change. Therefore, the random forest algorithm could compute the importance of each included variable. This procedure was conducted using Scikit-learn package (version 0.24.1) in Python 3.8.5.
A total of 156 patients were included in the final analysis. Table 1 shows the comparison of the clinical characteristics between the AL group and the control group. Compared to the controls, the patients in the AL group had longer operative time (225.03 ± 55.29 vs 207.17 ± 40.80, P = 0.023). Patients in the AL group exhibited lower levels of preoperative hemoglobin (123.32 vs 132.60, P = 0.003) and albumin (38.34 vs 40.52, P = 0.001), larger tumor size (4.07 vs 2.76, P < 0.001), and later cancer stage (P < 0.001) compared to the controls. The ASA score had a marginal effect (P = 0.049). No statistical difference was found between the AL group and the control group for other features.
| Variable | Controls (n = 78) | AL patients (n = 78) | P value |
| Male sex | 57 (71.3) | 57 (71.3) | 1.000 |
| Age, mean (SD), yr | 58.23 (9.46) | 56.82 (10.54) | 0.380 |
| Height, mean (SD), cm | 166.23 (7.92) | 166.87 (7.34) | 0.601 |
| Weight, mean (SD), kg | 63.41 (11.62) | 65.40 (11.39) | 0.282 |
| Operative time, mean (SD), min | 207.17 (40.80) | 225.03 (55.29) | 0.023 |
| Laparoscopic surgery | 77 (98.7) | 76 (97.4) | 1.000 |
| Location of tumor, mean (SD), cm | 7.86 (3.39) | 8.22 (3.59) | 0.507 |
| Intraperitoneal chemotherapy | 50 (64.1) | 54 (69.2) | 0.497 |
| Cleansing enema | 57 (73.1) | 60 (76.9) | 0.579 |
| Indwelling trans-anal tube | 73 (93.6) | 68 (87.2) | 0.174 |
| Indwelling drainage tube | 72 (92.3) | 74 (94.9) | 0.746 |
| Tumorous obstruction | 1 (1.3) | 6 (7.7) | 0.053 |
| Cigarette smoking | 24 (30.8) | 35 (44.9) | 0.098 |
| Alcohol use | 14 (17.9) | 21 (26.9) | 0.249 |
| Hypertension | 20 (25.6) | 19 (24.4) | 1.000 |
| Diabetes | 10 (12.8) | 11 (14.1) | 1.000 |
| Previous abdominal surgery | 11 (14.1) | 5 (6.4) | 0.186 |
| Preoperative antibiotics | 75 (76.2) | 72 (92.3) | 0.303 |
| Hemoglobin, mean (SD), g/L | 132.60 (16.31) | 123.32 (21.17) | 0.003 |
| Albumin, mean (SD), g/L | 40.52 (3.97) | 38.34 (4.01) | 0.001 |
| Neoadjuvant therapy | 1 (1.3) | 3 (3.8) | 0.620 |
| Tumor size, mean (SD), cm | 2.76 (1.28) | 4.07 (1.36) | < 0.001 |
| ASA score | 0.049 | ||
| 1 | 17 (21.86) | 9 (11.5) | |
| 2 | 56 (71.8) | 56 (71.8) | |
| 3 | 5 (6.4) | 13 (16.7) | |
| Stage | < 0.001 | ||
| 1 | 67 (85.9) | 19 (24.4) | |
| 2 | 5 (6.4) | 33 (42.3) | |
| 3 | 6 (7.7) | 26 (33.3) | |
| Number of linear stapler firings | 0.393 | ||
| 1 | 38(48.7) | 37 (47.4) | |
| 2 | 39 (50.0) | 37 (47.4) | |
| 3 | 1 (1.3) | 4 (5.1) | |
| Stoma | 20 (25.6) | 18 (23.1) | 0.852 |
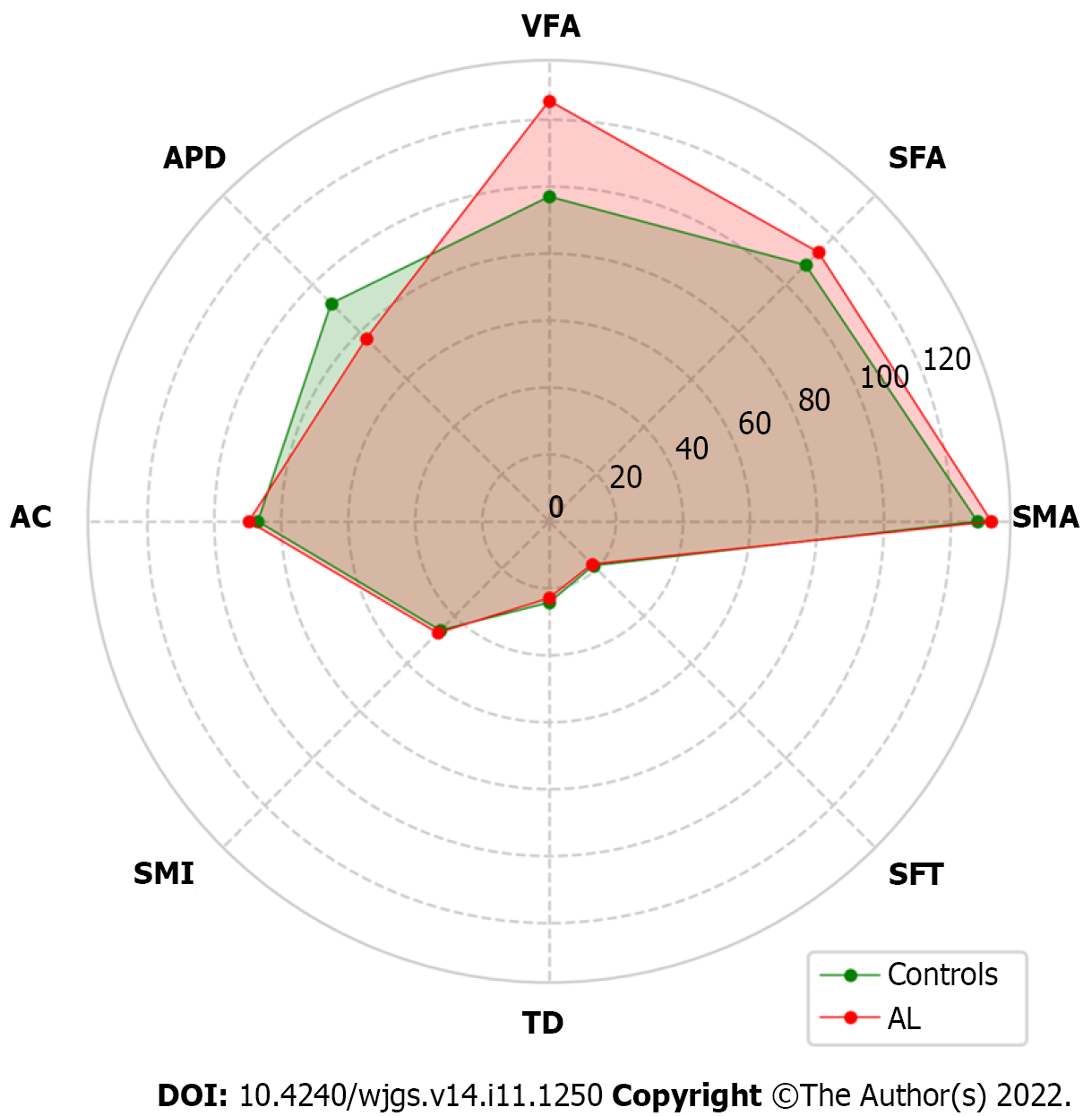
Table 2 shows the difference in abdominal composition related parameters between the AL group and the control group. Patients in the AL group had a larger VFA (125.68 vs 97.03, P = 0.008), a smaller APD (77.30 vs 92.09, P < 0.001), and a smaller TD (22.90 vs 24.21, P = 0.002) compared to those in the control group. These results are intriguing and suggest a potential contribution of a narrower abdominal cavity to AL development. Differences in other indicators were not statistically significant. A radar plot demonstrated the comparison of these indicators between the AL group and the control group (Figure 2).
| Variables | Controls (n = 78) | AL patients (n = 78) | P value |
| BMI (SD), kg/m2 | 23.05 (3.05) | 23.17 (2.88) | 0.797 |
| AC, mean (SD), cm | 87.00 (10.94) | 89.71 (14.20) | 0.120 |
| SFA, mean (SD), cm2 | 108.72 (54.12) | 113.72 (55.87) | 0.571 |
| SFT, mean (SD), mm | 18.68 (8.20) | 18.03 (7.31) | 0.601 |
| SMA, mean (SD), cm2 | 127.89 (29.57) | 132.06 (33.40) | 0.410 |
| SMI, mean (SD), cm2/m2 | 46.00 (8.81) | 47.10 (10.57) | 0.482 |
| VFA, mean (SD), cm2 | 97.03 (57.66) | 125.68 (73.59) | 0.008 |
| APD, mean (SD), mm | 92.09 (26.40) | 77.30 (23.23) | < 0.001 |
| TD, mean (SD), cm | 24.21 (2.90) | 22.90 (2.23) | 0.002 |
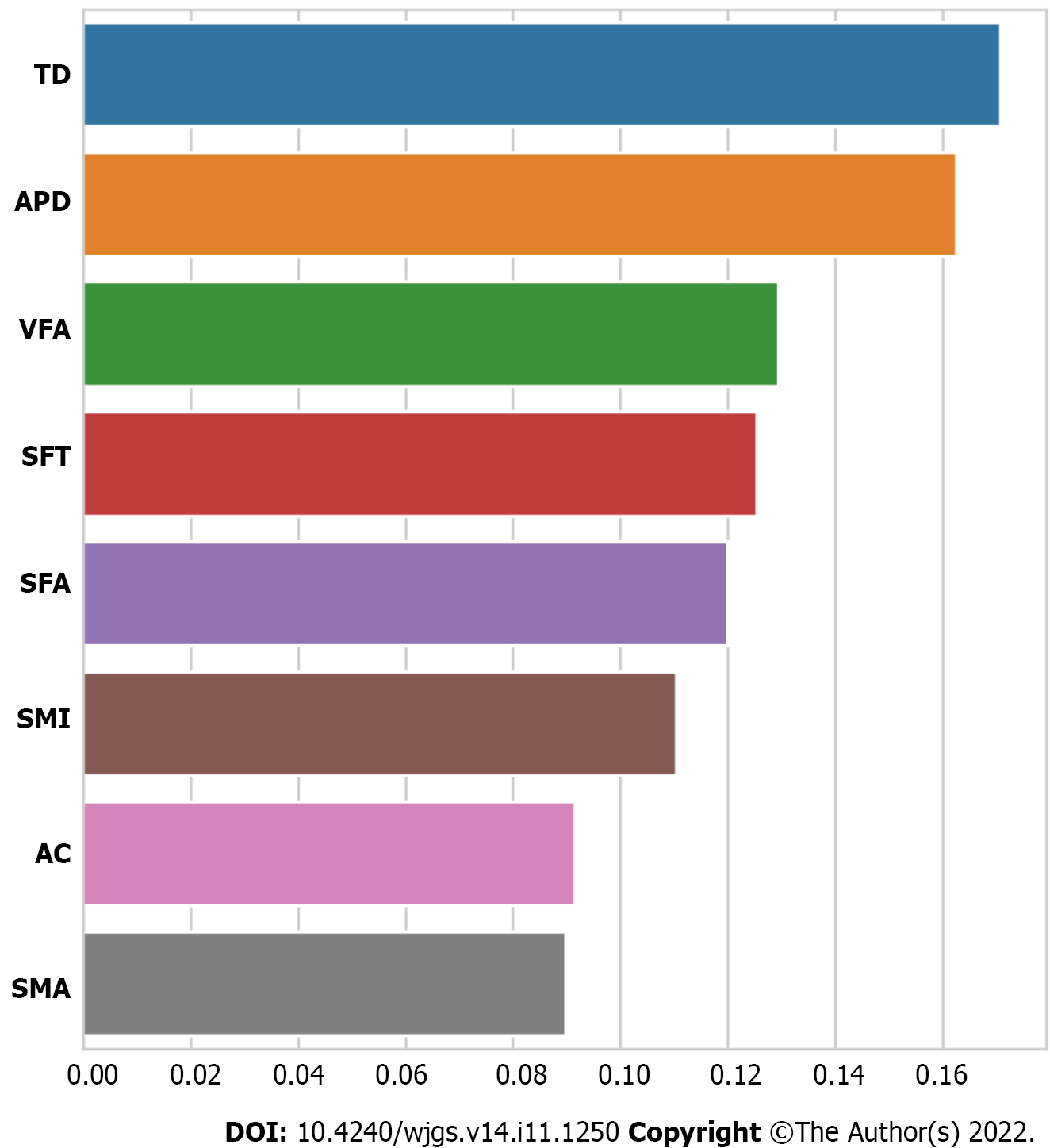
Although determination of statistical significance of abdominal composition-related indicators can be used to prove correlations, it is not sufficient. Feature importance analysis was conducted to quantify the contribution of each abdominal composition related indicator in AL development. Results demonstrated that TD, APD, and VFA were the three most important features (Figure 3). Additionally, we performed univariate and multivariate logistic regression analyses to investigate whether the VFA, APD, and TD were independent risk factors for AL. The data indicated that the VFA, APD, and TD were independent risk factors (P < 0.05) (Supplementary Table 1).
The mechanism underlying AL occurrence involves several factors. The present work compared the clinical characteristics and abdominal composition in rectal cancer patients who received anterior resection and developed AL to controls who were matched for sex and BMI. This study was conducted in a large academic hospital in which more than 4000 gastrointestinal operations were performed annually. Analysis revealed a 5.18% incidence of clinical AL, which concur with previous reports[21-23]. In this study cohort, when comparison was conducted in clinical characteristics, lower levels of preoperative hemoglobin and albumin, longer operative time, larger tumor size, and later cancer stage were associated with AL. In addition, when comparing abdominal composition related parameters, it is interesting to find that a higher visceral fat content and a narrower abdominal structure were associated with AL. This work provides evidence that the occurrence of AL is not only associated with patient related factors, but also with the underlying factors that may affect surgical technique.
Related studies have demonstrated that BMI, an easily available and most commonly used index of obesity, is a risk factor for AL in rectal cancer patients who received anterior resection. However, other studies have reported contrary reports[24,25]. Considering that BMI cannot distinguish between the content and distribution of fat and skeletal muscle, it is imperative to explore whether fat and skeletal muscle content or distribution potentially impacts the development of AL. Verduin et al[9] investigated the role of VFA on AL in 2370 colon cancer patients and the results implicated VFA as an independent risk factor for AL in the elective colon resection patients (odds ratio = 1.026, P = 0.035). Elsewhere, a study employed CT images to quantify the fat distribution and proposed the association of high adipose tissue with higher risk AL in rectal cancer patients[26]. However, whether VFA and other abdominal composition parameters potentially influence the occurrence of AL in patients with a similar BMI remains to be further evaluated. In addition, owing to the narrow pelvic structure, the male sex is widely accepted as an independent risk factor for AL in rectal cancer patients who received anterior resection, and some evidence has demonstrated the role of pelvic related parameters on AL[27]. Theoretically, a narrow pelvic structure is associated with the increased difficulty of the operation and prolonged operation time. All these features may increase the risk of AL. However, whether a narrow abdominal structure plays a similar role in AL occurrence is not known.
By comparing the differences in abdominal composition between AL and non-AL patients through sex and BMI matching, we found a higher VFA (125.68 vs 97.03, P = 0.008) and smaller narrow abdominal cavity structure (APD, 77.30 vs 92.09, P < 0.001; TD, 22.90 vs 24.21, P = 0.002) in AL patients than in the controls. The differences in skeletal muscle-related parameters, including SMA and SMI, were not significant, which may be ascribed to the unbalanced matching of other variables between the AL patients and controls, because various variables are associated with muscle content and density. This study provides support to the hypothesis that even with a similar BMI, AL patients are characterized by a higher VFA and a narrower abdominal structure.
This study has several limitations. First, as a single-center case-matched study, selection bias cannot be completely ignored. Second, although standard and strict screening and matching criteria were employed, the large initial sample size and the small sample size for analysis may imply that the research results need to be further validated on a larger cohort. Third, some variables impacting abdominal composition were not collected, including whether subjects are athletes, metabolic syndrome, etc. Lastly, this study was performed based on abdominal CT images, and as such, some indicators such as muscle density and intermuscular fat could not be evaluated in detail. Given the retrospective nature of this study and the small sample size, future longitudinal investigations with large samples are advocated to provide reliable data to determine causality for the correlation of abdominal components and AL.
The present analysis demonstrates the difference in abdominal components between AL patients and controls matched for sex and BMI. The contribution of each indicator to the development of AL was demonstrated. Intriguingly, in addition to the differences in VFA, the negative effects of APD and TD on AL were observed. This study adds considerable value to the field of AL preoperative risk assessment in rectal cancer patients. VFA, APD, and TD are potential indicators for predicting the risk of AL and can guide surgical decision-making (for example, performing a temporary ileostomy for high-risk patients).
Compelling evidence demonstrates the relationship of abdominal composition and postoperative complications. Anastomotic leakage (AL) is a fatal complication in patients with rectal cancer who have received anterior resection. However, the roles of abdominal composition on AL have not been studied.
To study the characteristics of abdominal components in patients who received rectal cancer surgery and developed AL.
To add risk factors for AL prediction in rectal cancer patients undergoing anterior resection for guiding surgical decision-making, e.g., performing a temporary ileostomy or not.
A retrospective case-matched cohort study was conducted. The abdominal composition was quantified based on computed tomography images by setting Hounsfield Unit thresholds. The abdominal composition related parameters were compared and the importance of these indicators was quantified using feature importance analysis.
A total of 156 cases were included in this study. Comparing the abdominal composition related parameters demonstrated that patients who developed AL exhibited a larger visceral fat area (VFA, 125.68 ± 73.59 vs 97.03 ± 57.66, P = 0.008) and a smaller anterior to posterior diameter of abdominal cavity (APD, 77.30 ± 23.23 vs 92.09 ± 26.40, P < 0.001) and transverse diameter of abdominal cavity (TD, 22.90 ± 2.23 vs 24.21 ± 2.90, P = 0.002). Feature importance analysis revealed TD, APD, and VFA to be the three most important abdominal composition related parameters.
Rectal cancer patients who have a higher visceral fat content and a narrower abdominal structure might be at a higher risk of developing AL.
A narrow abdominal structure is associated with the increased difficulty of the operation and prolonged operation time. In addition, the association of abdominal composition related parameters and postoperative complications was reported. But, whether abdominal composition is associated with AL is not known.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rama N, Portugal; Urlin VM, Romania S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Kitz J, Fokas E, Beissbarth T, Ströbel P, Wittekind C, Hartmann A, Rüschoff J, Papadopoulos T, Rösler E, Ortloff-Kittredge P, Kania U, Schlitt H, Link KH, Bechstein W, Raab HR, Staib L, Germer CT, Liersch T, Sauer R, Rödel C, Ghadimi M, Hohenberger W; German Rectal Cancer Study Group. Association of Plane of Total Mesorectal Excision With Prognosis of Rectal Cancer: Secondary Analysis of the CAO/ARO/AIO-04 Phase 3 Randomized Clinical Trial. JAMA Surg. 2018;153:e181607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Leonard D, Penninckx F, Fieuws S, Jouret-Mourin A, Sempoux C, Jehaes C, Van Eycken E; PROCARE, a multidisciplinary Belgian Project on Cancer of the Rectum. Factors predicting the quality of total mesorectal excision for rectal cancer. Ann Surg. 2010;252:982-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Miskovic D, Foster J, Agha A, Delaney CP, Francis N, Hasegawa H, Karachun A, Kim SH, Law WL, Marks J, Morino M, Panis Y, Uriburu JC, Wexner SD, Parvaiz A. Standardization of laparoscopic total mesorectal excision for rectal cancer: a structured international expert consensus. Ann Surg. 2015;261:716-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Law WL, Chu KW. Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg. 2004;240:260-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 365] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 5. | Artus A, Tabchouri N, Iskander O, Michot N, Muller O, Giger-Pabst U, Bourlier P, Bourbao-Tournois C, Kraemer-Bucur A, Lecomte T, Salamé E, Ouaissi M. Long term outcome of anastomotic leakage in patients undergoing low anterior resection for rectal cancer. BMC Cancer. 2020;20:780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Koedam TWA, Bootsma BT, Deijen CL, van de Brug T, Kazemier G, Cuesta MA, Fürst A, Lacy AM, Haglind E, Tuynman JB, Daams F, Bonjer HJ; COLOR COLOR II study group. Oncological Outcomes After Anastomotic Leakage After Surgery for Colon or Rectal Cancer: Increased Risk of Local Recurrence. Ann Surg. 2022;275:e420-e427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 7. | Denost Q, Rouanet P, Faucheron JL, Panis Y, Meunier B, Cotte E, Meurette G, Portier G, Sabbagh C, Loriau J, Benoist S, Piessen G, Sielezneff I, Lelong B, Mauvais F, Romain B, Barussaud ML, Capdepont M, Laurent C, Rullier E. Impact of early biochemical diagnosis of anastomotic leakage after rectal cancer surgery: long-term results from GRECCAR 5 trial. Br J Surg. 2021;108:605-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Kawada K, Sakai Y. Preoperative, intraoperative and postoperative risk factors for anastomotic leakage after laparoscopic low anterior resection with double stapling technique anastomosis. World J Gastroenterol. 2016;22:5718-5727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 77] [Cited by in F6Publishing: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Verduin WM, Warps AK, van den Helder R, Doodeman HJ, Houdijk APJ; INfluences of Fat And MUscle in colorectal Surgery Collaborative. Visceral Fat and Anastomotic Leakage After Colon Cancer Resection. Dis Colon Rectum. 2021;64:163-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Chen Z, Yang J, Liu Z, Zhang Y, Sun J, Wang P. Which obesity-associated parameters can better reflect the risk of the occurrence of the anastomotic leakage? Scand J Gastroenterol. 2020;55:466-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Daley BJ, Cecil W, Clarke PC, Cofer JB, Guillamondegui OD. How slow is too slow? J Am Coll Surg. 2015;220:550-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 206] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 12. | Thapa B, Sutanto E, Bhandari R. Thickness of subcutaneous fat is a risk factor for incisional surgical site infection in acute appendicitis surgery: a prospective study. BMC Surg. 2021;21:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Kim JH, Kim J, Lee WJ, Seong H, Choi H, Ahn JY, Jeong SJ, Ku NS, Son T, Kim HI, Han SH, Choi JY, Yeom JS, Hyung WJ, Song YG, Noh SH. A High Visceral-To-Subcutaneous Fat Ratio is an Independent Predictor of Surgical Site Infection after Gastrectomy. J Clin Med. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Zager Y, Khalilieh S, Ganaiem O, Gorgov E, Horesh N, Anteby R, Kopylov U, Jacoby H, Dreznik Y, Dori A, Gutman M, Nevler A. Low psoas muscle area is associated with postoperative complications in Crohn's disease. Int J Colorectal Dis. 2021;36:543-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Fujihata S, Ogawa R, Nakaya S, Hayakawa S, Okubo T, Sagawa H, Tanaka T, Takahashi H, Matsuo Y, Takiguchi S. The impact of skeletal muscle wasting during neoadjuvant chemotherapy on postoperative anastomotic leakage in patients with esophageal cancer. Esophagus. 2021;18:258-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Baracos VE, Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. 2018;29:ii1-ii9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 17. | Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 732] [Cited by in F6Publishing: 787] [Article Influence: 56.2] [Reference Citation Analysis (4)] |
| 18. | Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami T, Sato M, Uchino K, Enooku K, Kondo Y, Asaoka Y, Tanaka Y, Ohtomo K, Shiina S, Koike K. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 482] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 19. | Nachit M, Kwanten WJ, Thissen JP, Op De Beeck B, Van Gaal L, Vonghia L, Verrijken A, Driessen A, Horsmans Y, Francque S, Leclercq IA. Muscle fat content is strongly associated with NASH: A longitudinal study in patients with morbid obesity. J Hepatol. 2021;75:292-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 20. | McGovern J, Dolan RD, Horgan PG, Laird BJ, McMillan DC. Computed tomography-defined low skeletal muscle index and density in cancer patients: observations from a systematic review. J Cachexia Sarcopenia Muscle. 2021;12:1408-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 21. | Jang JH, Kim HC, Huh JW, Park YA, Cho YB, Yun SH, Lee WY, Yu JI, Park HC, Park YS, Park JO. Anastomotic Leak Does Not Impact Oncologic Outcomes After Preoperative Chemoradiotherapy and Resection for Rectal Cancer. Ann Surg. 2019;269:678-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 22. | Smith JD, Paty PB, Guillem JG, Temple LK, Weiser MR, Nash GM. Anastomotic leak is not associated with oncologic outcome in patients undergoing low anterior resection for rectal cancer. Ann Surg. 2012;256:1034-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Scarborough JE, Mantyh CR, Sun Z, Migaly J. Combined Mechanical and Oral Antibiotic Bowel Preparation Reduces Incisional Surgical Site Infection and Anastomotic Leak Rates After Elective Colorectal Resection: An Analysis of Colectomy-Targeted ACS NSQIP. Ann Surg. 2015;262:331-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 24. | Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, Kang SB, Kim JY, Kim BC, Bae BN, Son GM, Lee SI, Kang H. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013;257:665-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 25. | Ghuman A, Ganga R, Parisi Severino N, Krizzuk D, Li QZ, Wexner SD, Da Silva G. Clinical Factors Contributing to Anastomotic Leak After Mid-to-High Colorectal Anastomosis. Am Surg. 2021;31348211041555. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 26. | Nattenmüller J, Böhm J, Bagdassarjan A, Kulu Y, Gigic B, Schneider M, Kauczor HU, Ulrich CM, Ulrich A. CT-Quantified Adipose Tissue Distribution: Risk or Protective Factor for Complications after Rectal Cancer Surgery? Obes Facts. 2019;12:259-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Tsuruta A, Tashiro J, Ishii T, Oka Y, Suzuki A, Kondo H, Yamaguchi S. Prediction of Anastomotic Leakage After Laparoscopic Low Anterior Resection in Male Rectal Cancer by Pelvic Measurement in Magnetic Resonance Imaging. Surg Laparosc Endosc Percutan Tech. 2017;27:54-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |