Copyright
©The Author(s) 2023.
World J Gastrointest Surg. Sep 27, 2023; 15(9): 2032-2041
Published online Sep 27, 2023. doi: 10.4240/wjgs.v15.i9.2032
Published online Sep 27, 2023. doi: 10.4240/wjgs.v15.i9.2032
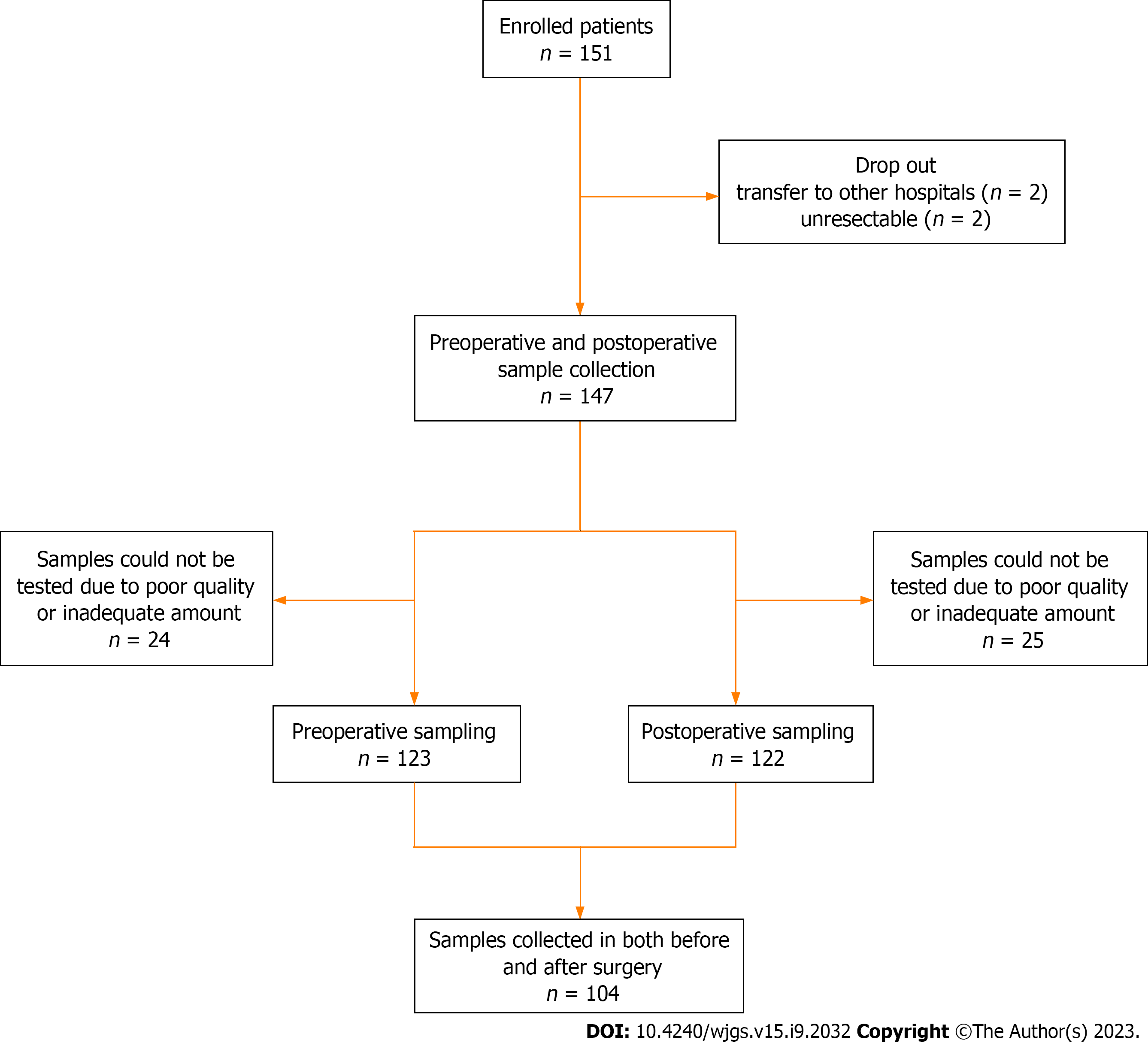
Figure 1
Flowchart of preoperative and postoperative sample collection in patients with colorectal cancer.
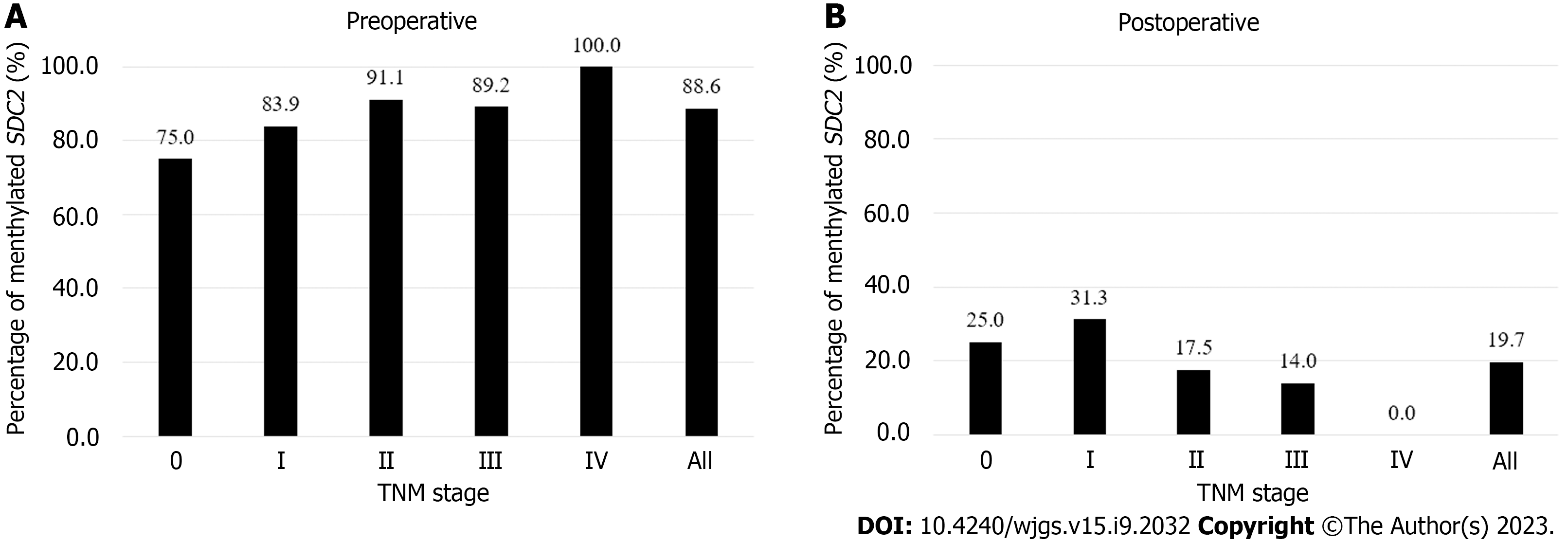
Figure 2 Percentages of syndecan-2 methylation test results according to tumor node metastasis stage.
Using the 1/2 algorithm, the percentage of samples with detectable methylated syndecan-2 is presented by bars. A: Preoperative stool samples were collected from 123 patients. Overall sensitivity was 88.6%; B: Postoperative stool samples were collected from 122 patients. Overall specificity was 80.3%. SDC2: Syndecan-2; TNM: Tumor node metastasis.
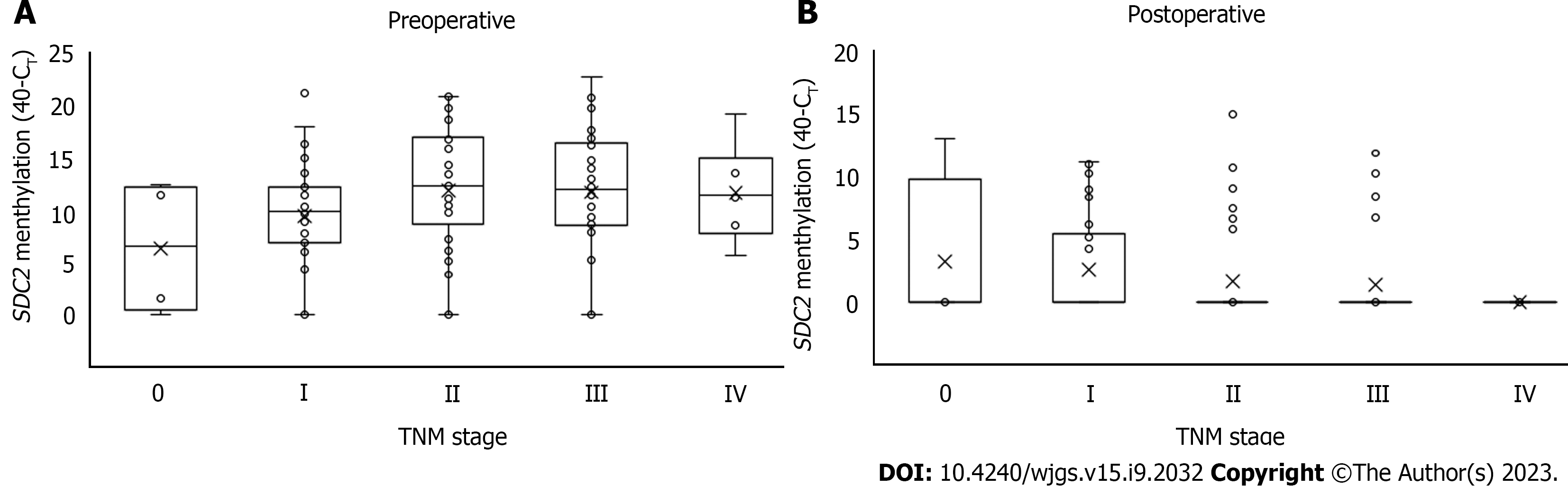
Figure 3 Distribution of syndecan-2 methylation according to tumor node metastasis stage.
The cycle threshold (CT) values for each sample were calculated as 40-CT. A higher 40-CT indicates a higher methylation level of syndecan-2 (SDC2). If SDC2 methylation was not detected, it was expressed as 0. A: Preoperative (n = 123); B: Postoperative (n = 122). SDC2: Syndecan-2; CT: Cycle threshold; TNM: Tumor node metastasis.
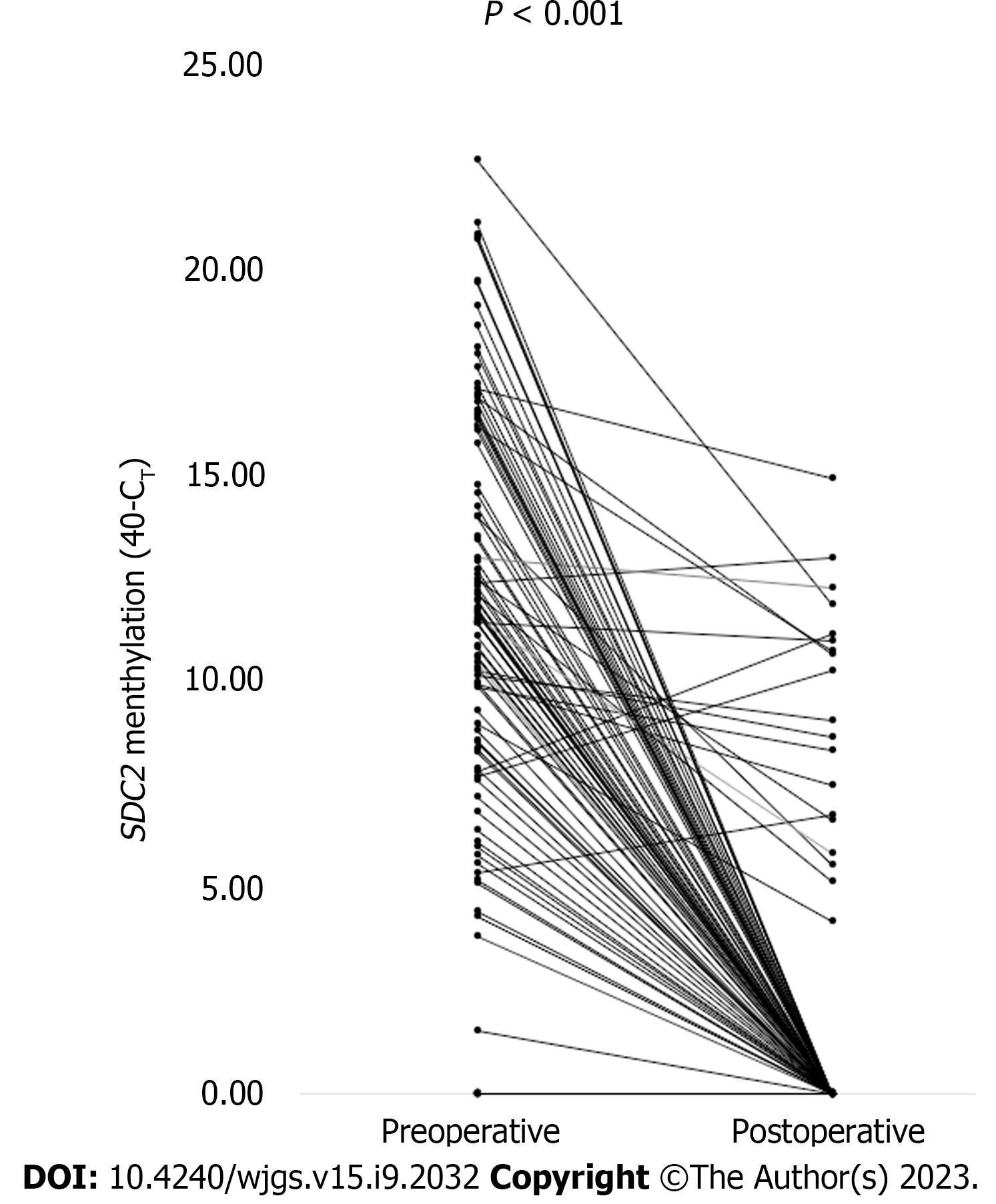
Figure 4 Paired cycle threshold values of syndecan-2 methylation before and after surgery for each patient (n = 104).
The cycle threshold (CT) values were calculated as 40-CT. A higher 40-CT indicated a higher methylation level of syndecan-2 (SDC2). If SDC2 methylation was not detected, it was expressed as 0. P value was calculated using a paired t-test. SDC2: Syndecan-2; CT: Cycle threshold.
- Citation: Song JH, Oh TJ, An S, Lee KH, Kim JY, Kim JS. Comparative detection of syndecan-2 methylation in preoperative and postoperative stool DNA in patients with colorectal cancer. World J Gastrointest Surg 2023; 15(9): 2032-2041
- URL: https://www.wjgnet.com/1948-9366/full/v15/i9/2032.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i9.2032