Copyright
©The Author(s) 2017.
World J Diabetes. Feb 15, 2017; 8(2): 45-55
Published online Feb 15, 2017. doi: 10.4239/wjd.v8.i2.45
Published online Feb 15, 2017. doi: 10.4239/wjd.v8.i2.45
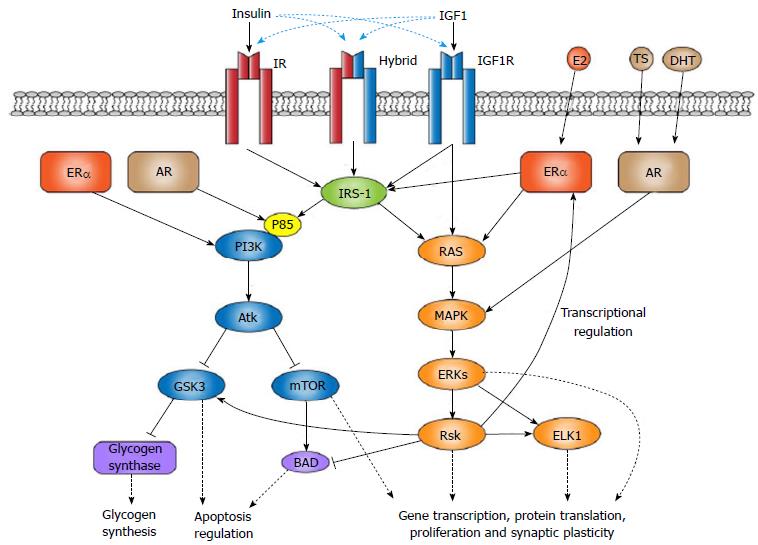
Figure 1 Similar signaling cascades involved with neuroprotection for insulin-like peptides and sex hormones.
The insulin receptor (IR), insulin-like growth factor 1 receptor (IGF1R), and insulin-IGF1 hybrid receptor enact their neuroprotection through the mitogen-activated protein kinases-extracellular signal-related kinase (MAPK-ERK) or phosphoinositide 3-kinase (PI3K)-Akt pathways signaling cascades. Although IGF1R can directly activate the RAS-ERK pathway, both the insulin-like peptide receptors and the estrogen receptor alpha (ERα) firstly interact with insulin receptor substrate 1 (IRS-1) scaffolding proteins. ERα and the androgen receptor (AR) can also directly modulate PI3K-Akt and MAPK-ERK signaling. Both IRS-1 and p85 binding of PI3K are increased with ERα activation, leading to downstream Akt-derived inhibition of glycogen synthase kinase 3 (GSK3) and mammalian target of rapamycin (mTOR). GSK3, specifically, is involved with glycogen synthesis, while both effectors are involved in apoptosis. A similar effect may occur with AR’s ability to modulate p85 binding to PI3K. AR-induced MAPK-ERK signaling also results in ribosomal S6 kinase (Rsk) expression that can inhibit the pro-apoptosis bcl-2-associated death promoter protein, as well as effects on the ER, GSK3, and the ETS-like transcription factor, ELK1. Solid black arrows indicate downstream interaction. Dashed black arrows represent the influence of kinases or proteins on the cellular environment. Dashed blue arrows represent the binding capabilities of IGF1 and insulin across all three receptor types.
- Citation: Huffman J, Hoffmann C, Taylor GT. Integrating insulin-like growth factor 1 and sex hormones into neuroprotection: Implications for diabetes. World J Diabetes 2017; 8(2): 45-55
- URL: https://www.wjgnet.com/1948-9358/full/v8/i2/45.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i2.45